Pro-Den Rx
Pro-Den Rx
FULL PRESCRIBING INFORMATION
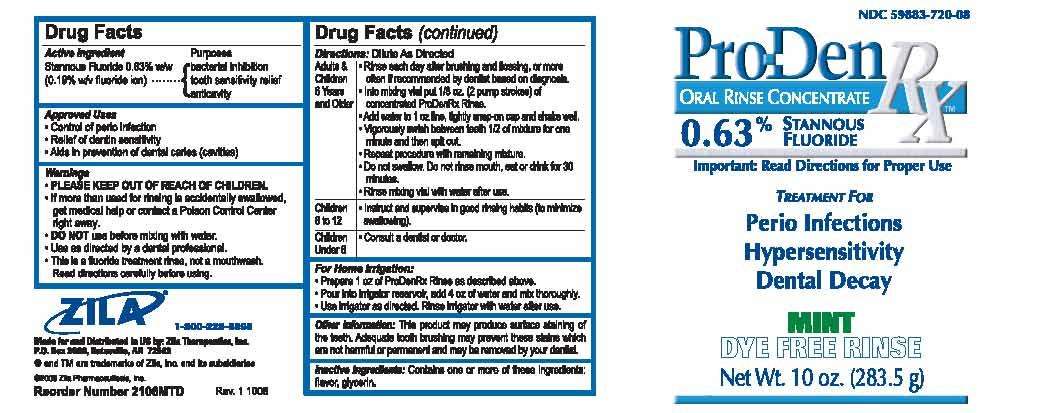
Drug Facts
Stannous Fluoride 0.63% w/w (0.19% w/v fluoride ion)
Bacterial inhibition
Tooth sensitivity relief
Anticavity
Approved Uses
- Control of perio infection
- Relief of dentin sensitivity
- Aids in prevention of dental caries (cavities).
- PLEASE KEEP OUT OF REACH OF CHILDREN.
- If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- DO NOT use before mixing with water.
- Use as directed by a dental professional.
- This is a fluoride treatment rinse, not a mouthwash. Read directions carefully before using.
Directions: Dilute As Directed
| Adults and Children 6 years and Older |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Children 6 to 12 |
|
| Children Under 6 |
|
For Home Irrigation:
- Prepare 1 oz of ProDenRex Rinse as described above.
- Pour into irrigator reservoir, add 4 oz of water and mix thoroughly.
- Use irrigator as directed. Rinse irrigator with water after use.
Other information: This product may produce surface staining of the teeth. Adequate tooth brushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
Contains one or more of these ingredients: flavor, glycerin
Made for and Distributed in US by:
Zila Therapeutics, Inc.,
P.O. Box 3889, Batesville, AR 72503
1-800-228-5595
Reorder Number 2106CI D
Rev. 1 1008

Pro-Den RxSodium Fluoride GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||