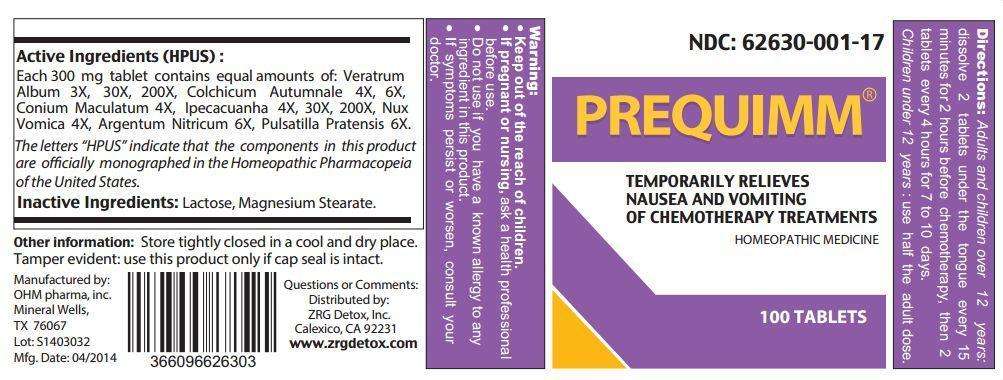
PREQUIMM
ZRG DETOX, INC.
OHM PHARMA INC.
PREQUIMM
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients (HPUS):
Each 300 mg tablet contains equal amounts of: Veratrum Album 3X, 30X, 200X, Colchicum Autumnale 4X, 6X, Conium Maculatum 4X, Ipecacuanha 4X, 30X, 200X, Nux Vomica 4X, Argentum Nitricum 6X, Pulsatila Pratensis 6X.
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopeia of the United States.
Inactive Ingredients: Lactose, Magnesium Stearate.
Directions:
- Adults and children over 12 years: dissolve 2 tablets under the tongue every 15 minutes for 2 hours before chemotherapy, then 2 tablets every 4 hours for 7 to 10 days.
- Children under 12 years: use half the adult dose.
Warning:
- Keep out of the reach of children.
- If pregnant or nursing, ask a health professional before use.
- Do not use if you have a known allergy to any ingredient in this product.
- If symptoms persist or worsen, consult your doctor.
Keep out of the reach of children.
Uses
Temporarily relieves nasea and vomiting of chemotherapy treatments.
Other information: Store tightly closed in a cool and dry place. Tamper evident: use this product only if cap seal is intact.
Questions or Comments:
Distributed by: ZRG Detox, Inc.
Calexico, CA 92231
www.zrgdetox.com
Manufactured by:
OHM pharma, inc.
Mineral Wells, TX 76067
NDC: 62630-001-17
PREQUIMM
HOMEOPATHIC MEDICINE
100 TABLETS
Purpose
TEMPORARILY RELIEVES NAUSEA AND VOMITING OF CHEMOTHERAPY TREATMENTS.
PREQUIMMVeratrum Album, Colchicum Autumnale, Conium Maculatum, Ipecacuanha, Nux Vomica, Argentum Nitricum, Pulsatilla Pratensis TABLET, ORALLY DISINTEGRATING
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||