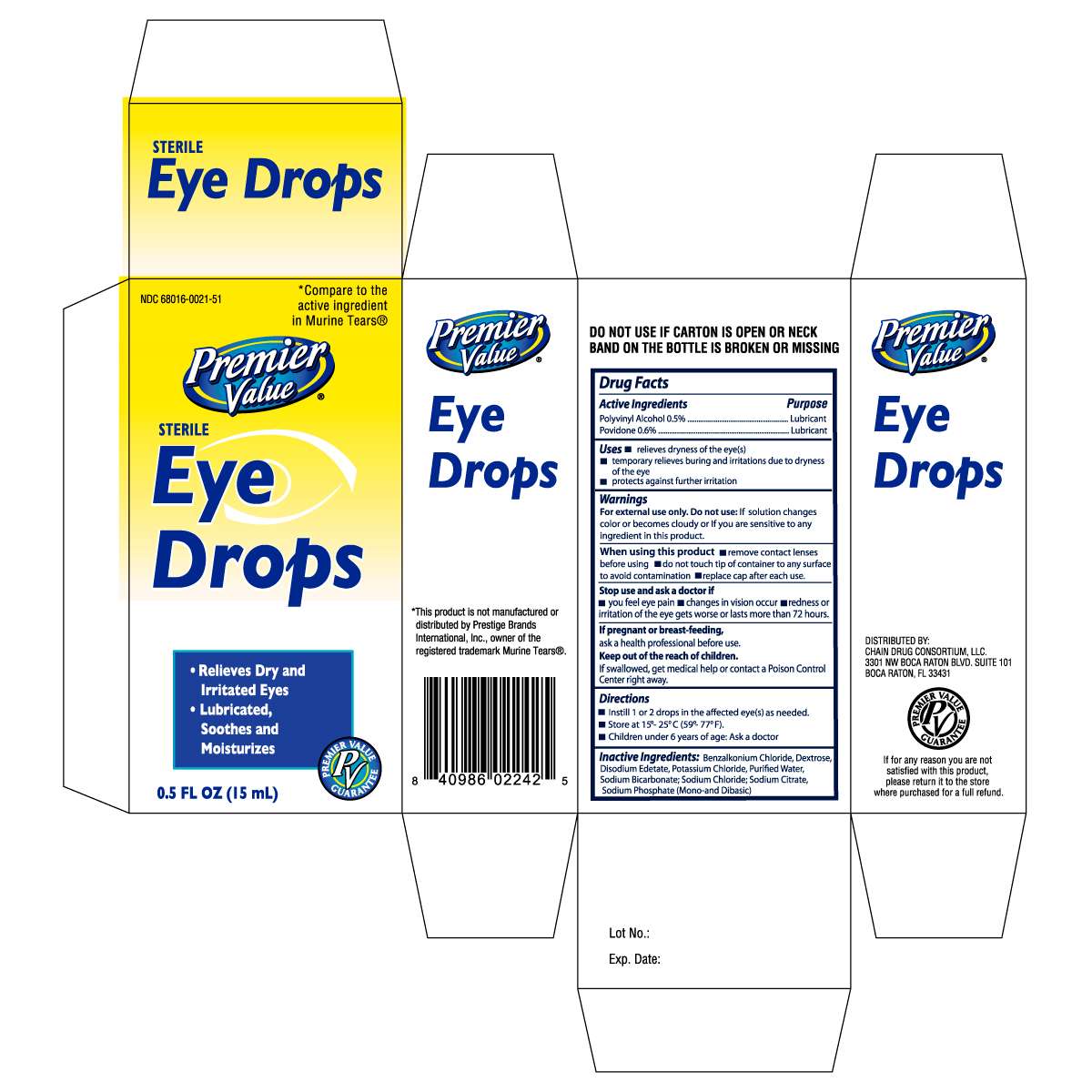
PREMIER VALUE EYE DROPS MT
HANLIM PHARM. CO., LTD.
UNITED EXCHANGE CORP.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients Purpose
Polyvinyl Alcohol 0.5% ........................................................Lubricant
Povidone 0.6% ...................................................................Lubricant
Purpose
Uses
- relieves dryness of the eye(s)
- temporary relieves burning and irritations due to dryness of the eye
- protects against further irritation
For external use only.
Do not use: If solution changes color or becomes cloudy or if you are sensitive to any ingredient in this product.
When using this product
- remove contact lenses before using
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur
- redness of irritation of the eye gets worse or lasts more than 72 hours
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of the reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
Directions
- Instill 1 or 2 drops in the affected eye(s) as needed.
- Store at 15o-25oC (59o-77oF).
- Children under 6 years of age: Ask a doctor
Inactive Ingredients: Benzalkonium Chloride, Dextrose, Disodium Edetate, Potassium Chloride, Purified Water, Sodium Bicarbonate, Sodium Chloride, Sodium Citrate,
Sodium Phosphate (Mono- and Dibasic)
Distributed By:
Chain Drug Consortium, LLC.
3301 NW Boca Raton Blvd. Suite 101
Boca Raton, FL33431
 Enter section text here
Enter section text here
PREMIER VALUE EYE DROPS MTPOLYVINYL ALCOHOL AND POVIDONE SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||