Pravastatin Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRAVASTATIN SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- PRAVASTATIN SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PRAVASTATIN SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PRAVASTATIN SODIUM DESCRIPTION

CLINICAL PHARMACOLOGY
Clinical Studies
PHARMACOKINETICS
CLINICAL STUDIES
Prevention of Coronary Heart Disease: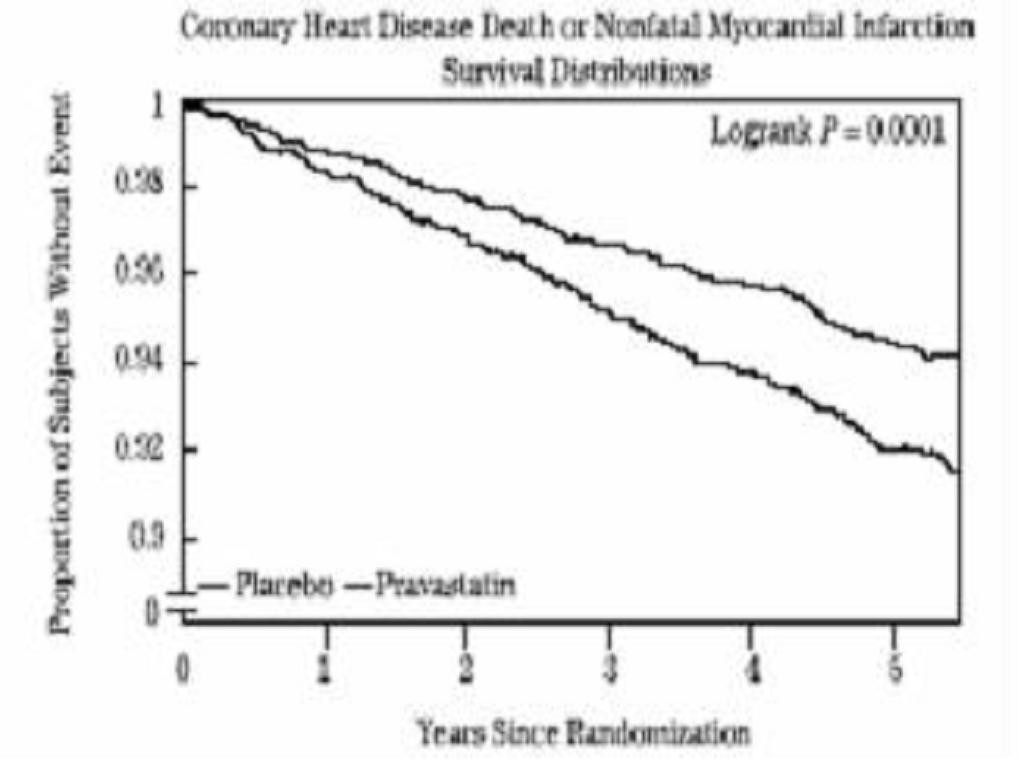
Secondary Prevention of Cardiovascular Events:
Primary Hypercholesterolemia (Fredrickson Type IIa and IIb):
Table 1
Table 1
Table 1
**
Table 2
Dysbetalipoproteinemia (Fredrickson Type III):
Pediatric Clinical Study:
Table 4
*
*
INDICATIONS & USAGE
Primary Prevention of Coronary Events:
Hyperlipidemia:
NCEP Guidelines
The National Cholesterol Education Program's Treatment Guidelines are summarized below:
*CHD*or CHD risk equivalents (10-year risk > 20%)<100(100 to129: drug optional)2+ Risk factors (10- year risk20%)< 13010-year risk 10% to 20%: (13010-year risk < 10%:1600 to 1 Risk factor<160(160 to 189: LDL- lowering drug optional)After the LDL-C goal has been achieved, if the TG is still200 mg/dL, non-HDL-C (Total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C is130 mg/dL (seeNCEP Guidelines, above).
Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the Total-C be used to monitor therapy.
As with other lipid-lowering therapy, pravastatin sodium tablets are not indicated when hypercholesterolemia is due to hyperalphalipoproteinemia (elevated HDL-C).
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below:
CategoryTotal-C (mg/dL)LDL-C (mg/dL)Acceptable<170<110Borderline170 to 199110 to 129High
PRAVASTATIN SODIUM CONTRAINDICATIONS
WARNINGS
Pregnancy and Lactation:
Pregnancy
WARNINGS
Liver Enzymes:CLINICAL PHARMACOLOGYClinical Studiestimes the upper limit of normal for those with normal ALT (the upper limit of normal) at baseline or a doubling of the baseline ALT for those with elevated ALT (> the upper limit of normal) at baseline. By Week 36, 12 out of 160 (7.5%) subjects treated with pravastatin met the prespecified safety ALT endpoint compared to 20 out of 160 (12.5%) subjects receiving placebo. Conclusions regarding liver safety are limited since the study was not large enough to establish similarity between groups (with 95% confidence) in the rates of ALT elevation.
It is recommended that liver function tests be performed prior to the initiation of therapy, prior to the elevation of the dose, and when otherwise clinically indicated.
Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of pravastatin (seeCONTRAINDICATIONS). Caution should be exercised when pravastatin is administered to patients who have a recent (< 6 months) history of liver disease, have signs that may suggest liver disease (e.g., unexplained aminotransferase elevations, jaundice), or are heavy users of alcohol (seeCLINICAL PHARMACOLOGY:Pharmacokinetics/Metabolism). Such patients should be closely monitored, started at the lower end of the recommended dosing range (seeDOSAGE AND ADMINISTRTION), and titrated to the desired therapeutic effect.
Patients who develop increased transaminase levels or signs and symptoms of active liver disease while taking pravastatin should be evaluated with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality (ies) return to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawal of pravastatin therapy is recommended.
Skeletal Muscle:
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with pravastatin and other drugs in this class. Uncomplicated myalgia has also been reported in pravastatin-treated patients (seeADVERSE REACTIONS
The risk of myopathy during treatment with another HMG-CoA reductase inhibitor is increased with concurrent therapy with either erythromycin, cyclosporine, niacin, or fibrates. However, neither myopathy nor significant increases in CPK levels have been observed in three reports involving a total of 100 post-transplant patients (24 renal and 76 cardiac) treated for up to two years concurrently with pravastatin 10 to 40 mg and cyclosporine. Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and niacin, there were no reports of myopathy. Also, myopathy was not reported in a trial of combination pravastatin (40 mg/day) and gemfibrozil (1200 mg/day), although 4 of 75 patients on the combination showed marked CPK elevations versus one of 73 patients receiving placebo. There was a trend toward more frequent CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups receiving placebo, gemfibrozil, or pravastatin monotherapy (see PRECAUTIONS :Drug Interactions). The use of fibrates alone may occasionally be associated with myopathy. The combined use of pravastatin and fibrates should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination.
PRECAUTIONS
General:ADVERSE REACTIONS
Homozygous Familial Hypercholesterolemia:
Renal Insufficiency:
INFORMATION FOR PATIENTS
Skeletal MuscleDRUG INTERACTIONS
Skeletal MuscleCytochrome P450 3A4 Inhibitors:
Diltiazem:
Itraconazole:
Antipyrine:
Cholestyramine/Colestipol:
Concomitant Therapy
Warfarin:
Cimetidine:
Digoxin:
Cyclosporine:
Gemfibrozil:
Skeletal Muscle
Endocrine Function:
CNS Toxicity:
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category X.CONTRAINDICATIONS
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
Pediatric PatientsAdult PatientsPediatric PatientsGERIATRIC USE
Pharmacokinetics/Metabolism
PRAVASTATIN SODIUM ADVERSE REACTIONS
Geriatric UseAdverse Clinical Events:
Short-Term Controlled Trials:
****
Long -Term Controlled Morbidity and Mortality Trials:
Post-marketing Experience:
Laboratory Test Abnormalities:
WARNINGS
Concomitant Therapy:
Skeletal MuscleDrug Interactions
Pediatric Patients:
Pediatric Clinical StudyPediatric Use
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
NCEP Treatment GuidelinesAdult Patients:
Pediatric Patients:
Children (Ages 8 to 13 Years, Inclusive):
Adolescents (Ages 14 to 18 Years):
NCEP Treatment Guidelines
Skeletal Muscle
Concomitant Therapy:
Concomitant Therapy
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Pravastatin SodiumPravastatin Sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!