Power Relief
POWERRELIEF™ COOLING PAIN RELIEF ROLL ON
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Indications
- Warnings
- Directions
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 59.1 ml Bottle Label
FULL PRESCRIBING INFORMATION
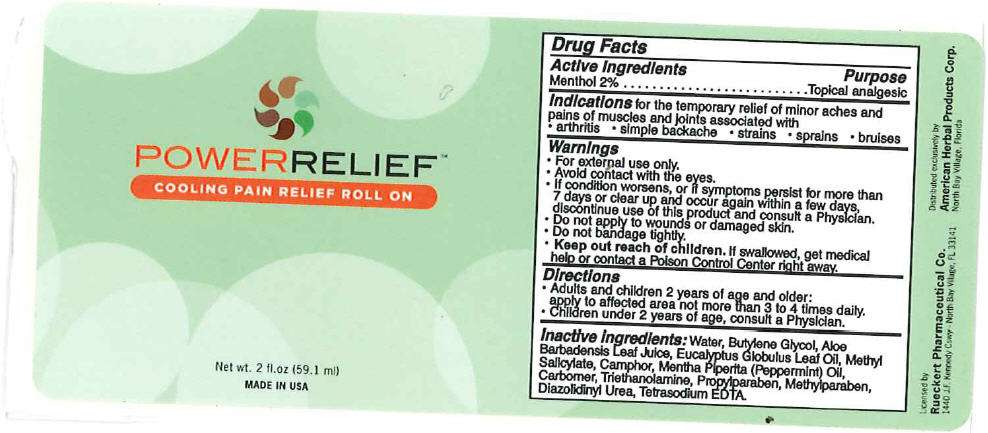
Drug Facts
Active Ingredients
Menthol 2%
Purpose
Topical analgesic
Indications
for the temporary relief of minor aches and pains of muscles and joints associated with
- arthritis
- simple backache
- strains
- sprains
- bruises
Warnings
- For external use only.
- Avoid contact with the eyes.
- If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a Physician.
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
- Keep out reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Adults and children 2 years of age and older:
apply to affected area not more than 3 to 4 times daily. - Children under 2 years of age, consult a Physician.
Inactive Ingredients
Water, Butylene Glycol, Aloe Barbadensis Leaf Juice, Eucalyptus Globulus Leaf Oil, Methyl Salicylate, Camphor, Mentha Piperita (Peppermint) Oil, Carbomer, Triethanolamine, Propylparaben, Methylparaben, Diazolidinyl Urea, Tetrasodium EDTA.
Distributed exclusively by
American Herbal Products Corp.
North Bay Village, Florida
PRINCIPAL DISPLAY PANEL - 59.1 ml Bottle Label
POWERRELIEF™
COOLING PAIN RELIEF ROLL ON
Net wt. 2 fl.oz (59.1 ml)
MADE IN USA
Power ReliefMenthol GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!