Povidone-Iodine
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Povidone-Iodine Uses
- Warnings
- Directions
- Povidone-Iodine Other information
- Inactive ingredients
- Questions
- Principal Display Panel - Swabstick Singles pouch
- Principal Display Panel - Swabstick Singles box
- Drug Facts Panel - Swabstick Singles box
- Principal Display Panel - Swabstick Triples box
- Drug Facts Panel - Swabstick Triples box
- Principal Display Panel - Prep Pads, Medium box
- Drug Facts Panel - Prep Pads, Medium box
- Principal Display Panel - Prep Pad Bulk case
- Drug Facts Panel - Prep Pad Bulk case
- Principal Display Panel - Prep Solution bottle
- Principal Display Panel - Prep Solution label
- Principal Display Panel - Prep Solution label
FULL PRESCRIBING INFORMATION
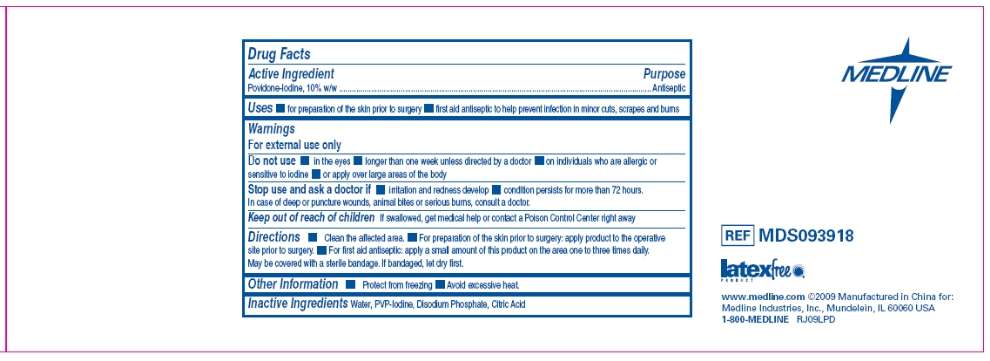
Active ingredient
Povidone Iodine 10% (equivalent to 1% available iodine)
Purpose
First Aid Antiseptic
Povidone-Iodine Uses
- Healthcare antiseptic for preparation of the skin prior to surgery
- First aid antiseptic to help prevent infection in minor cuts, scrapes and burns
Warnings
For external use only.
Do not use
- in the eyes
- longer than 1 week unless directed by a doctor
- on individuals who are allergic or sensitive to iodine
- or apply over large areas of the body
Stop use and ask a doctor
- if irritation and redness develop
- if condition persists for more than 72 hours
- in case of deep or puncture wounds, animal bites or serious burns
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Clean the affected area.
- For preparation of the skin prior to surgery: apply product to the operative site prior to surgery
- For first aid antiseptic: apply a small amount of this product on the area 1 to 3 times daily. May be covered with a sterile bandage. If bandaged, let dry first.
Povidone-Iodine Other information
Protect from freezing. Avoid excessive heat.
Inactive ingredients
Citric Acid, Disodium Phosphate, Nonoxynol-9, Sodium Hydroxide, Water.
Questions
1-800-MEDLINE
Principal Display Panel - Swabstick Singles pouch

NDC53329-945-09
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Singles
MDS093901
latex free
For professional and hospital use.
Contents: 1 Swabstick
Principal Display Panel - Swabstick Singles box

NDC: 53329-945-29
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Singles
MDS093901
Discard appropriately after single use.
Protect from Freezing. Avoid excessive heat. For external use only.
latex free
Contents: 50 Each
Drug Facts Panel - Swabstick Singles box

Principal Display Panel - Swabstick Triples box

NDC 53329-946-75
Medline
Antiseptic
Povidone-Iodine, USP
Swabsticks, Triples
MDS093902
Discard appropriately after single use.
Protect from freezing. Avoid excessive heat. For external use only.
latex free
Contents: 25 Pouches
Drug Facts Panel - Swabstick Triples box

Principal Display Panel - Prep Pads, Medium box
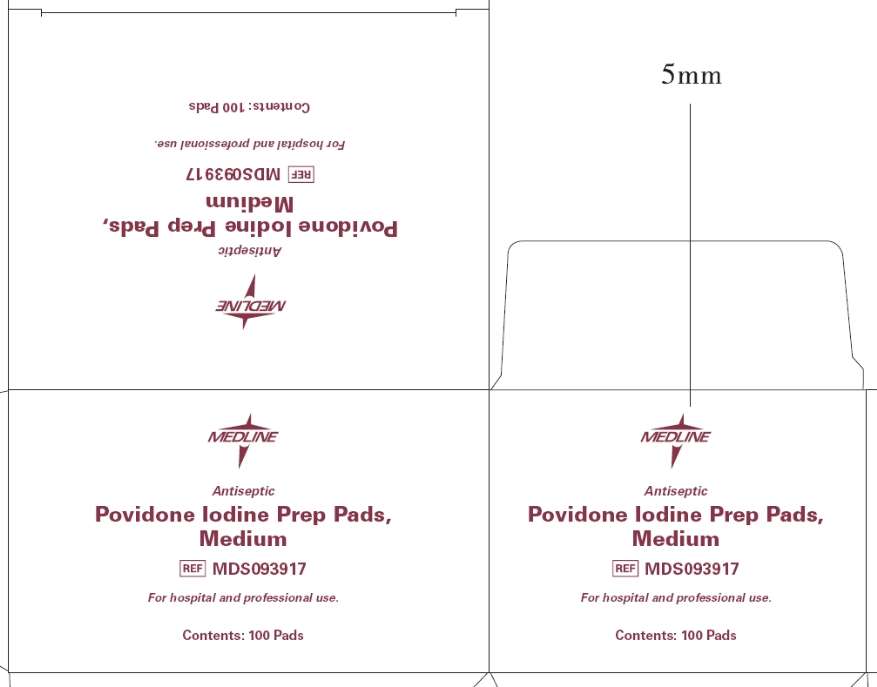
Medline
Antiseptic
Povidone Iodine Prep Pads, Medium
MDS093917
For hospital and professional use.
Contents: 100 Pads
Drug Facts Panel - Prep Pads, Medium box

Principal Display Panel - Prep Pad Bulk case

Povidone Iodine
Prep PADS
Medium Bulk
MDS093918
Contents: 3,000 Each Per Case
Drug Facts Panel - Prep Pad Bulk case
Principal Display Panel - Prep Solution bottle

NDC: 53329-939-13
MEDLINE
PVP Prep Solution
Topical Antiseptic
Povidone-Iodine 10%
MDS093940
latexfree
www.medline.com
Manufactured for: Medline Industries, Inc.
Mundelein, IL 60060 USA Made in USA
To Order Call: 1-800-MEDLINE
RB11CTR
2 FL OZ (59 mL)
Principal Display Panel - Prep Solution label

N: 53329-900-55
Medline
Prep Solution
with
Spray Bottle
Topical Antiseptic
Microbicide
MDS093942
latex free
Do not reuse
2 FL OZ (59 mL)
Principal Display Panel - Prep Solution label

NDC 53329-904-04
Medline
Prep Solution
with Spray Bottle
Povidone Iodine USP, 10% Topical Solution
MDS093934
Topical Antiseptic
4 FL Oz (118 ml)
Povidone-IodinePovidone-Iodine SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Povidone-IodinePovidone-Iodine SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Povidone-IodinePovidone-Iodine SWAB
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Povidone-IodinePovidone-Iodine SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Povidone-IodinePovidone-Iodine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prep SolutionPovidone-Iodine SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prep SolutionPovidone-Iodine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||