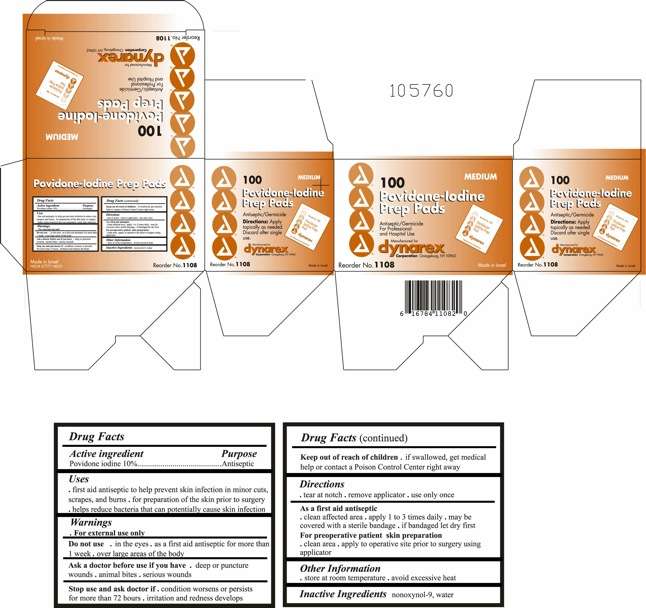
povidine iodine
Dynarex Corporation
Dynarex Corporation
Povidone Iodine Prep Pad
FULL PRESCRIBING INFORMATION: CONTENTS*
- povidine iodine Uses:
- Warnings:
- Do not use:
- Ask a doctor before use if you have:
- Stop Use:
- Keep Out Of Reach Of Children
- Directions:
- povidine iodine Other information:
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Section Text
Active Ingredient Purpose
Povidone Iodine 10% v/v Antiseptic
Uses:
- First aid antiseptic to help prevent skin infection in minor cuts, scrapes and burns.
- For preparation of the skin prior to surgery.
- Helps reduce bacteria that can potentially cause skin infections.
Warnings:
Section Text
-
FOR EXTERNAL USE ONLY
Do not use:
Section Text
- As a first aid antiseptic for more than 1 week.
- In the eyes.
- Over large areas of the body.
Ask a doctor before use if you have:
- Deep puncture wounds
- Animal bites
- Serious burns
Stop Use:
- If irritation and redness develop
- If condition persists for more than 72 hours, consult a physician.
Keep Out Of Reach Of Children
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center.
Directions:
Tear at notch, remove applicator, use only once.
As a first aid antiseptic
- clean affected area
- apply 1 to 3 times daily
- may be covered with a sterile bandage, if bandaged let dry.
For preoperative patient skin preparation
- clean area
- apply to operative site prior to surgery using the applicator
Other information:
Store at room temperature.
Avoid excessive heat
Principal Display Panel
Principal Display Panel
povidine iodinepovidine iodine SWAB
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||