Potassium Chloride in Sodium Chloride
INTRAVENOUS SOLUTIONS with POTASSIUM CHLORIDE
FULL PRESCRIBING INFORMATION: CONTENTS*
- POTASSIUM CHLORIDE IN SODIUM CHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- POTASSIUM CHLORIDE IN SODIUM CHLORIDE INDICATIONS AND USAGE
- POTASSIUM CHLORIDE IN SODIUM CHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- POTASSIUM CHLORIDE IN SODIUM CHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- POTASSIUM CHLORIDE IN SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
- INSTRUCTIONS FOR USE
- HOW SUPPLIED
- IM-0477
- IM-0478
FULL PRESCRIBING INFORMATION
Potassium Chloride in 0.9% Sodium Chloride Injection, USP
|
Flexible Plastic Container |
Rx only |
POTASSIUM CHLORIDE IN SODIUM CHLORIDE DESCRIPTION
Intravenous solutions with potassium chloride (I.V. solutions with KCl) are sterile and nonpyrogenic solutions in water for injection. They are for administration by intravenous infusion only.
See Table for summary of content and characteristics of these solutions.
The solutions contain no bacteriostat, antimicrobial agent or added buffer and each is intended only for use as a single-dose injection. When smaller doses are required the unused portion should be discarded.
These solutions are parenteral fluid and/or electrolyte replenishers.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Water for Injection, USP is chemically designated H20.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of its chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
CLINICAL PHARMACOLOGY
When administered intravenously, these solutions provide a source of water and potassium chloride with 0.9% sodium chloride.
Intravenous solutions containing potassium chloride are particularly intended to provide needed potassium cation (K+). Potassium is the chief cation of body cells (160 mEq/liter of intracellular water). It is found in low concentration in plasma and extracellular fluids (3.5 to 5.0 mEq/liter in a healthy adult). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine; the remainder in the stools and to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues resulting in potassium depletion. A deficiency of either potassium or chloride will lead to a deficit of the other.
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl¯) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl¯) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl¯) are largely under the control of the kidney which maintains a balance between intake and output.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
POTASSIUM CHLORIDE IN SODIUM CHLORIDE INDICATIONS AND USAGE
These solutions are indicated in patients requiring parenteral administration of potassium chloride and sodium chloride.
POTASSIUM CHLORIDE IN SODIUM CHLORIDE CONTRAINDICATIONS
Solutions containing potassium chloride are contraindicated in diseases where high potassium levels may be encountered.
WARNINGS
Solutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
To avoid potassium intoxication, do not infuse these solutions rapidly. In patients with severe renal insufficiency or adrenal insufficiency, administration of potassium chloride may cause potassium intoxication.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
The intravenous administration of these solutions can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentration of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Potassium replacement therapy should be guided primarily by serial electrocardiograms. Plasma potassium levels are not necessarily indicative of tissue potassium levels.
High plasma concentrations of potassium may cause death through cardiac depression, arrhythmias or arrest.
Potassium-containing solutions should be used with caution in the presence of cardiac disease, particularly in digitalized patients or in the presence of renal disease.
Care should be exercised to insure that the needle (or catheter) is well within the lumen of the vein and that extravasation does not occur.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy Category C: Animal reproduction studies have not been conducted with potassium chloride or sodium chloride. It is also not known whether potassium chloride or sodium chloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Potassium chloride or sodium chloride should be given to a pregnant woman only if clearly needed.
Pediatric Use
The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
POTASSIUM CHLORIDE IN SODIUM CHLORIDE ADVERSE REACTIONS
Reactions which may occur because of the solutions or technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Nausea, vomiting, abdominal pain and diarrhea have been reported with potassium therapy. The signs and symptoms of potassium intoxication include paresthesias of the extremities, flaccid paralysis, listlessness, mental confusion, weakness and heaviness of the legs, hypotension, cardiac arrhythmias, heart block, electrocardiographic abnormalities such as disappearance of P waves, spreading and slurring of the QRS complex with development of a biphasic curve and cardiac arrest.
Potassium-containing solutions are intrinsically irritating to tissues. Therefore, extreme care should be taken to avoid perivascular infiltration. Local tissue necrosis and subsequent sloughing may result if extravasation occurs. Chemical phlebitis and venospasm have also been reported.
Should perivascular infiltration occur, I.V. administration at that site should be discontinued at once. Local infiltration of the affected area with procaine hydrochloride, 1%, to which hyaluronidase may be added, will often reduce venospasm and dilute the potassium remaining in the tissues locally. Local application of heat may also be helpful.
OVERDOSAGE
In the event of potassium overdosage, discontinue the infusion immediately and institute intensive corrective therapy to reduce serum potassium levels. (See WARNINGS and PRECAUTIONS .)
POTASSIUM CHLORIDE IN SODIUM CHLORIDE DOSAGE AND ADMINISTRATION
These solutions should be administered only by intravenous infusion and as directed by the physician. The dose and rate of injection are dependent upon the age, weight and clinical condition of the patient. If the serum potassium level is greater than 2.5 mEq/liter, potassium should be given at a rate not to exceed 10 mEq/hour in a concentration less than 30 mEq/liter. Somewhat faster rates and greater concentrations (usually up to 40 mEq/liter) of potassium may be indicated in patients with more severe potassium deficiency. The total 24-hour dose should not generally exceed 200 mEq of potassium.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See PRECAUTIONS .)
INSTRUCTIONS FOR USE
To Open
Tear outer wrap at notch and remove solution container. If supplemental medication is desired, follow directions below before preparing for administration. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
To Add Medication
-
Prepare additive port.
-
Using aseptic technique and an additive delivery needle of appropriate length, puncture resealable additive port at target area, inner diaphragm and inject. Withdraw needle after injecting medication.
-
The additive port may be protected by covering with an additive cap.
-
Mix container contents thoroughly.
Preparation for Administration
(Use aseptic technique)
-
Close flow control clamp of administration set.
-
Remove cover from outlet port at bottom of container.
-
Insert piercing pin of administration set into port with a twisting motion until the set is firmly seated. NOTE: See full directions on administration set carton.
-
Suspend container from hanger.
-
Squeeze and release drip chamber to establish proper fluid level in chamber.
-
Open flow control clamp and clear air from set. Close clamp.
-
Attach set to venipuncture device. If device is not indwelling, prime and make venipuncture.
-
Regulate rate of administration with flow control clamp.
WARNING: Do not use flexible container in series connections.
HOW SUPPLIED
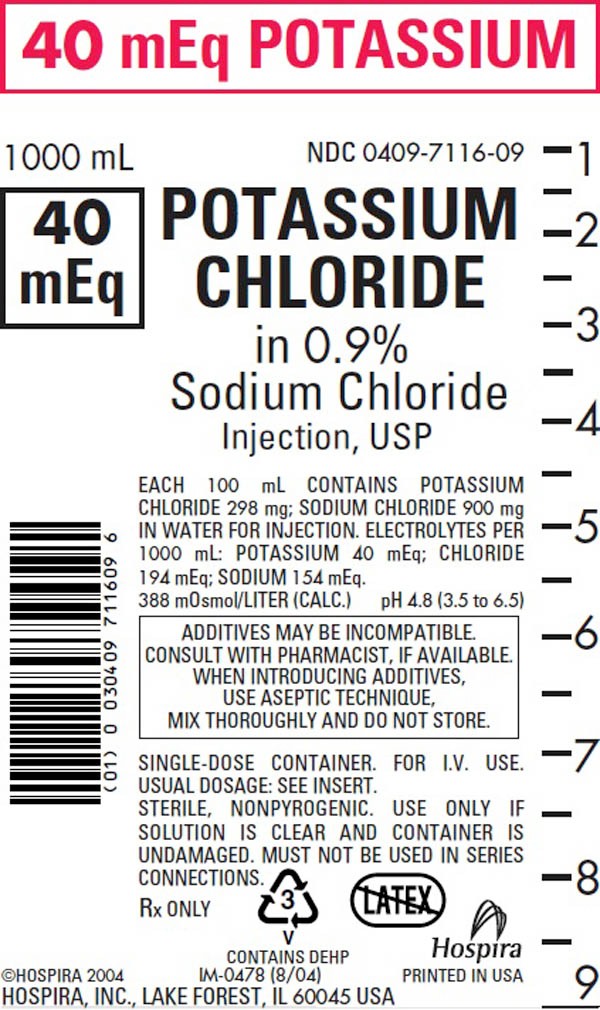
Intravenous solutions with potassium chloride (I.V. solution with KCl) are supplied in single-dose flexible plastic containers. See Table:
|
Potassium Chloride in |
||||||||||
|
0.9% Sodium Chloride |
||||||||||
|
Inj., USP |
COMPOSITION |
Approx. Ionic Concentrations |
||||||||
|
(g/L) |
Calculated |
(mEq/L) |
||||||||
|
NDC No. |
mEq Potassium |
Size (mL) |
Sodium Chloride |
Potassium Chloride |
Osmolarity (mOsmol/L) |
pH (range) |
Sodium (Na + ) |
Potassium (K + ) |
Chloride (Cl¯) |
Approximate kcal/L |
|
0409–7115–09 |
20 mEq |
1000 |
9 |
1.49 |
348 |
4.8 (3.5 to 6.5) |
154 |
20 |
174 |
0 |
|
0409–7116–09 |
40 mEq |
1000 |
9 |
2.98 |
388 |
4.8 (3.5 to 6.5) |
154 |
40 |
194 |
0 |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: April, 2009
|
Printed in USA |
EN-2102 |
Hospira, Inc., Lake Forest, IL 60045 USA
IM-0477

IM-0478
Potassium Chloride in Sodium ChlorideSODIUM CHLORIDE and POTASSIUM CHLORIDE INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Potassium Chloride in Sodium ChlorideSODIUM CHLORIDE and POTASSIUM CHLORIDE INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||