Pleo Muc
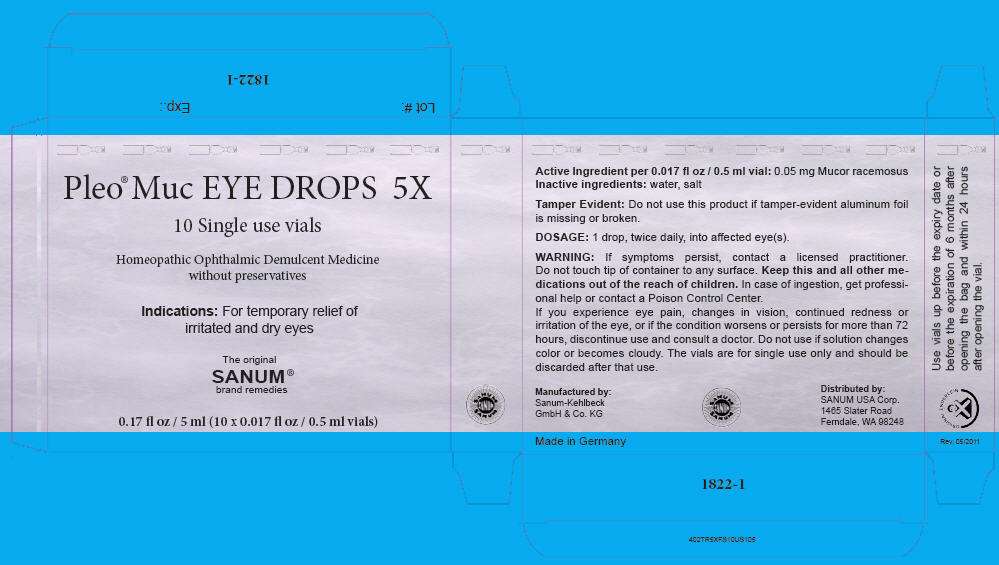
Pleo Muc EYE DROPS 5X
FULL PRESCRIBING INFORMATION: CONTENTS*
- Indications
- Active Ingredient per 0.017 fl oz / 0.5 ml vial
- Inactive ingredient
- Tamper Evident
- DOSAGE
- WARNING
- PRINCIPAL DISPLAY PANEL - 5 ml Carton
FULL PRESCRIBING INFORMATION
Purpose
Homeopathic Ophthalmic Demulcent Medicine without preservatives
Indications
For temporary relief of irritated and dry eyes
Active Ingredient per 0.017 fl oz / 0.5 ml vial
0.05 mg Mucor racemosus
Inactive ingredient
water, salt
Tamper Evident
Do not use this product if tamper-evident aluminum foil is missing or broken.
DOSAGE
1 drop, twice daily, into affected eye(s).
WARNING
If symptoms persist, contact a licensed practitioner.
Do not touch tip of container to any surface.
Keep this and all other medications out of the reach of children. In case of ingestion, get professional help or contact a Poison Control Center.
If you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours, discontinue use and consult a doctor.
Do not use if solution changes color or becomes cloudy. The vials are for single use only and should be discarded after that use.
Distributed by:
SANUM USA Corp.
1465 Slater Road
Ferndale, WA 98248
Manufactured by:
Sanum-Kehlbeck
GmbH & Co. KG
PRINCIPAL DISPLAY PANEL - 5 ml Carton
Pleo® Muc EYE DROPS 5X
10 Single use vials
Homeopathic Ophthalmic Demulcent Medicine
without preservatives
Indications: For temporary relief of
irritated and dry eyes
The original
SANUM
®
brand remedies
0.17 fl oz / 5 ml (10 x 0.017 fl oz / 0.5 ml vials)
Pleo Mucmucor racemosus LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||