Pioglitazone Hydrochloride and Metformin Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use pioglitazone hydrochloride and metformin hydrochloride tablets safely and effectively. See full prescribing information for pioglitazone hydrochloride and metformin hydrochloride tablets. Pioglitazone Hydrochloride and Metformin Hydrochloride Tablets for Oral UseInitial U.S. Approval: 2005RECENT MAJOR CHANGES(2.1)(2.2)(2.3)(5.4)(5.6)BOXED WARNINGWARNING: CONGESTIVE HEART FAILURE AND LACTIC ACIDOSIS See full prescribing information for complete boxed warning Congestive Heart Failure Thiazolidinediones, including pioglitazone, which is a component of pioglitazone hydrochloride and metformin hydrochloride, cause or exacerbate congestive heart failure in some patients. (5.1) After initiation of pioglitazone hydrochloride and metformin hydrochloride, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride and metformin hydrochloride must be considered. (5.1) Pioglitazone hydrochloride and metformin hydrochloride is not recommended in patients with symptomatic heart failure. Initiation of pioglitazone hydrochloride and metformin hydrochloride in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. (4, 5.1) Lactic Acidosis Lactic acidosis can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic impairment, renal impairment, and acute congestive heart failure. (5.2) Symptoms include malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap, and elevated blood lactate. (5.2) If acidosis is suspected, discontinue pioglitazone hydrochloride and metformin hydrochloride and hospitalize the patient immediately. (5.2) INDICATIONS AND USAGE(114) Not for treatment of type 1 diabetes or diabetic ketoacidosis. (1.2) DOSAGE AND ADMINISTRATION Individualize the starting dose of pioglitazone hydrochloride and metformin hydrochloride tablets based on the patient’s current regimen. (2) May adjust the dosing based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of pioglitazone 45 mg and metformin 2550 mg. (2) Maximum recommended dose of pioglitazone is 15 mg once daily in patients taking strong CYP2C8 inhibitors (e.g., gemfibrozil). (2.3, 7.1) Pioglitazone hydrochloride and metformin hydrochloride tablets should be given in divided daily doses with meals to reduce the gastrointestinal (GI) side effects due to metformin. (2) Dose increases should be accompanied by careful monitoring for adverse events related to fluid retention. (2) Obtain liver tests before starting pioglitazone hydrochloride and metformin hydrochloride tablets. If abnormal, use caution when treating with pioglitazone hydrochloride and metformin hydrochloride tablets, investigate the probable cause, treat (if possible) and follow appropriately. Monitoring liver tests while on pioglitazone hydrochloride and metformin hydrochloride tablets are not recommended in patients without liver disease. (5.4) DOSAGE FORMS AND STRENGTHS(3)CONTRAINDICATIONS Do not initiate pioglitazone hydrochloride and metformin hydrochloride tablets in patients with established NYHA Class III or IV heart failure. (4) Do not use in patients with a history of a serious hypersensitivity reaction to pioglitazone hydrochloride and metformin hydrochloride tablets or its ingredients. (4) Renal impairment. Metabolic acidosis, including diabetic ketoacidosis. (4, 5.2) WARNINGS AND PRECAUTIONS Congestive heart failure: Fluid retention may occur and can exacerbate or lead to congestive heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk. Monitor patients for signs and symptoms. (5.1) Edema: Dose-related edema may occur. (5.3) Lactic acidosis: Warn against excessive alcohol intake. Pioglitazone hydrochloride and metformin hydrochloride is not recommended in hepatic impairment and is contraindicated in renal impairment. Ensure normal renal function before initiating and at least annually thereafter. (5.2) Temporarily discontinue in patients undergoing radiologic studies with intravascular iodinated contrast materials or any surgical procedures necessitating restricted intake of food and fluids. (5.12) Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt pioglitazone hydrochloride and metformin hydrochloride and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart pioglitazone hydrochloride and metformin hydrochloride if liver injury is confirmed and no alternate etiology can be found. (5.4) Fractures: Increased incidence in female patients. Apply current standards of care for assessing and maintaining bone health. (5.5) Bladder cancer: Preclinical and clinical trial data, and results from an observational study suggest an increased risk of bladder cancer in pioglitazone users. The observational data further suggest that the risk increases with duration of use. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer. (5.6) Hypoglycemia: When used with insulin or an insulin secretagogue, a lower dose of the insulin or insulin secretagogue may be needed to reduce the risk of hypoglycemia. (5.7) Macular edema: Postmarketing reports. Recommend regular eye exams in all patients with diabetes according to current standards of care with prompt evaluation for acute visual changes. (5.8) Vitamin B12 deficiency: Metformin may lower vitamin B12 levels. Monitor hematologic parameters annually. (5.14) Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone hydrochloride and metformin hydrochloride or any other anti-diabetic drug. (5.15) Side Effects(6.1)To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Strong CYP2C8 inhibitors (e.g., gemfibrozil) increase pioglitazone concentrations. Limit pioglitazone hydrochloride and metformin hydrochloride dose to 15 mg/850 mg daily. (2.3, 7.1) CYP2C8 inducers (e.g., rifampin) may decrease pioglitazone concentrations. (7.2) Cationic drugs: May reduce metformin elimination. Use with caution in patients who are taking cationic medications eliminated by renal tubular secretion. (7.4) USE IN SPECIFIC POPULATIONS(8.3)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: CONGESTIVE HEART FAILURE AND LACTIC ACIDOSIS
- 1 PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE INDICATIONS AND USAGE
- 2 PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Congestive Heart Failure
- 5.2 Lactic Acidosis
- 5.3 Edema
- 5.4 Hepatic Effects
- 5.5 Fractures
- 5.6 Urinary Bladder Tumors
- 5.7 Hypoglycemia
- 5.8 Macular Edema
- 5.9 Ovulation
- 5.10 Monitoring of Renal Function
- 5.11 Hypoxic States
- 5.12 Surgical Procedures
- 5.13 Alcohol Intake
- 5.14 Vitamin B12 Levels
- 5.15 Macrovascular Outcomes
- 6 PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 PIOGLITAZONE HYDROCHLORIDE AND METFORMIN HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.2 FDA-Approved Medication Guide
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg/500 mg (60 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg/850 mg (60 Tablet Bottle)
FULL PRESCRIBING INFORMATION
WARNING: CONGESTIVE HEART FAILURE AND LACTIC ACIDOSIS
Congestive Heart Failure
- Thiazolidinediones, including pioglitazone, which is a component of pioglitazone hydrochloride and metformin hydrochloride, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.1)].
- After initiation of pioglitazone hydrochloride and metformin hydrochloride, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride and metformin hydrochloride must be considered [see Warnings and Precautions (5.1)].
- Pioglitazone hydrochloride and metformin hydrochloride is not recommended in patients with symptomatic heart failure.
- Initiation of pioglitazone hydrochloride and metformin hydrochloride in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)].
Lactic Acidosis
- Lactic acidosis is a rare, but serious complication that can occur due to metformin accumulation. The risk increases with conditions such as sepsis, dehydration, excess alcohol intake, hepatic impairment, renal impairment, and acute congestive heart failure [see Warnings and Precautions (5.2)].
- The onset is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. Laboratory abnormalities include low pH, increased anion gap and elevated blood lactate [see Warnings and Precautions (5.2)].
- If acidosis is suspected, pioglitazone hydrochloride and metformin hydrochloride should be discontinued and the patient hospitalized immediately [see Warnings and Precautions (5.2)].
1 INDICATIONS AND USAGE
Pioglitazone hydrochloride and metformin hydrochloride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both pioglitazone and metformin is appropriate [see Clinical Studies (14)].
1.2 Important Limitations of Use
Pioglitazone exerts its antihyperglycemic effect only in the presence of endogenous insulin. Pioglitazone hydrochloride and metformin hydrochloride tablets should not be used to treat type 1 diabetes or diabetic ketoacidosis, as they would not be effective in these settings.
Use caution in patients with liver disease [see Warnings and Precautions (5.4)].
2 DOSAGE AND ADMINISTRATION
2.1 Recommendations for All Patients
If therapy with a combination tablet containing pioglitazone and metformin is considered appropriate the recommended starting dose is:
- 15 mg/500 mg twice daily or 15 mg/850 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients with New York Heart Association (NYHA) Class I or Class II congestive heart failure: 15 mg/500 mg or 15 mg/850 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on metformin monotherapy: 15 mg/500 mg twice daily or 15 mg/850 mg once or twice daily (depending on the dose of metformin already being taken) and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on pioglitazone monotherapy: 15 mg/500 mg twice daily or 15 mg/850 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability.
- for patients who are changing from combination therapy of pioglitazone plus metformin as separate tablets: Pioglitazone hydrochloride and metformin hydrochloride tablets should be taken at doses that are as close as possible to the dose of pioglitazone and metformin already being taken.
Pioglitazone hydrochloride and metformin hydrochloride tablets may be titrated up to a maximum daily dose of 45 mg of pioglitazone and 2550 mg of metformin.
Metformin doses above 2000 mg may be better tolerated given 3 times a day.
After initiation of pioglitazone hydrochloride and metformin hydrochloride tablets or with dose increase, monitor patients carefully for adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure [see Boxed Warning and Warnings and Precautions (5.1)]. Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating pioglitazone hydrochloride and metformin hydrochloride tablets. Routine periodic monitoring of liver tests during treatment with pioglitazone hydrochloride and metformin hydrochloride tablets are not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of pioglitazone hydrochloride and metformin hydrochloride tablets or who are found to have abnormal liver tests while taking pioglitazone hydrochloride and metformin hydrochloride tablets should be managed as described under Warnings and Precautions [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
2.2 Concomitant Use with an Insulin Secretagogue or Insulin
If hypoglycemia occurs in a patient co-administered pioglitazone hydrochloride and metformin hydrochloride tablets and an insulin secretagogue (e.g., sulfonylurea), the dose of the insulin secretagogue should be reduced.
If hypoglycemia occurs in a patient co-administered pioglitazone hydrochloride and metformin hydrochloride tablets and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
2.3 Coadministration with Strong CYP2C8 Inhibitors
Coadministration of pioglitazone (one of the ingredients in pioglitazone hydrochloride and metformin hydrochloride tablets) and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone hydrochloride and metformin hydrochloride tablets is 15 mg/850 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
- 15 mg/500 mg tablets: White to off-white, oblong, biconvex film-coated tablets, debossed with ‘H’ on one side and ‘92’ on other side.
- 15 mg/850 mg tablets: White to off-white, oblong, biconvex film-coated tablets, debossed with ‘H’ on one side and ‘93’ on other side.
4 CONTRAINDICATIONS
[see Boxed Warning]
- Renal impairment (e.g., serum creatinine levels ≥ 1.5 mg/dL [males], ≥ 1.4 mg/dL [females], or abnormal creatinine clearance) which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia [see Warnings and Precautions (5.2, 5.10)].
- Known hypersensitivity to pioglitazone, metformin or any other component of pioglitazone hydrochloride and metformin hydrochloride tablets.
- Metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin.
5 WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure
Pioglitazone
[see Boxed Warning Contraindications (4), and Adverse Reactions (6.1)]
5.2 Lactic Acidosis
Metformin Hydrochloride
Lactic Acidosis
[see Warnings and Precautions (5.10, 5.11, 5.12, 5.13)][see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]
[see Warnings and Precautions (5.10)]
[see Contraindications (4) and Warnings and Precautions (5.10)]
5.3 Edema
[see Adverse Reactions (6.1)]
[see Boxed Warning Warnings and Precautions (5.1) and Patient Counseling Information (17.1)]
5.4 Hepatic Effects
There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone controlled clinical trial database to date [see Adverse Reactions (6.1)].
Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) and assessing the patient is recommended before initiating pioglitazone hydrochloride and metformin hydrochloride therapy.
In patients with abnormal liver tests, pioglitazone hydrochloride and metformin hydrochloride should be initiated with caution.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than three times the upper limit of the reference range), pioglitazone hydrochloride and metformin hydrochloride treatment should be interrupted and investigation done to establish the probable cause. Pioglitazone hydrochloride and metformin hydrochloride should not be restarted in these patients without another explanation for the liver test abnormalities.
Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury, and should not be restarted on pioglitazone hydrochloride and metformin hydrochloride. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with pioglitazone hydrochloride and metformin hydrochloride can be used with caution.
Because impaired hepatic function has been associated with some cases of lactic acidosis pioglitazone hydrochloride and metformin hydrochloride should generally be avoided in patients with clinical or laboratory evidence of hepatic disease.
5.5 Fractures
5.6 Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In two 3-year trials in which pioglitazone was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo.
A five-year interim report of an ongoing 10-year observational cohort study found a nonsignificant increase in the risk for bladder cancer in subjects ever exposed to pioglitazone, compared to subjects never exposed to pioglitazone (HR 1.2 [95% CI 0.9 to 1.5]). Compared to never exposure, a duration of pioglitazone therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 to 2.1]), which reached statistical significance after more than 24 months of pioglitazone use (HR 1.4 [95% CI 1.03 to 2]). Interim results from this study suggested that taking pioglitazone longer than 12 months increased the relative risk of developing bladder cancer in any given year by 40% which equates to an absolute increase of 3 cases in 10,000 (from approximately 7 in 10,000 [without pioglitazone] to approximately 10 in 10,000 [with pioglitazone]).
There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors. Consequently, pioglitazone hydrochloride and metformin hydrochloride should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with pioglitazone hydrochloride and metformin hydrochloride should be considered in patients with a prior history of bladder cancer.
5.7 Hypoglycemia
[see Dosage and Administration (2.2)]
5.8 Macular Edema
[see Adverse Reactions (6.1)]
5.9 Ovulation
[see Use in Specific Populations (8.1)]
5.10 Monitoring of Renal Function
Use of concomitant medications that may affect renal function or metformin disposition
[see Clinical Pharmacology (12.3)]
Radiological studies and surgical procedures
[see Contraindications (4)]
5.11 Hypoxic States
5.12 Surgical Procedures
5.13 Alcohol Intake
5.14 Vitamin B12 Levels
12 12 1212 1212 12
5.15 Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone hydrochloride and metformin hydrochloride or any other oral anti-diabetic drug.
6 ADVERSE REACTIONS
- Congestive heart failure [see Boxed Warning and Warnings and Precautions (5.1)]
- Lactic acidosis [see Boxed Warning and Warnings and Precautions (5.2)]
- Edema [see Warnings and Precautions (5.3)]
- Fractures [see Warnings and Precautions (5.5)]
6.1 Clinical Studies Experience
Pioglitazone
Common Adverse Events: 16 to 26-Week Monotherapy Trials
| % of Patients | ||
|---|---|---|
| Placebo N=259 |
Pioglitazone N=606 |
|
| Upper Respiratory Tract Infection |
8.5 |
13.2 |
| Headache |
6.9 |
9.1 |
| Sinusitis |
4.6 |
6.3 |
| Myalgia |
2.7 |
5.4 |
| Pharyngitis |
0.8 |
5.1 |
Common Adverse Events: 16 to 24-Week Add-on Combination Therapy Trials
| 16-Week Placebo-Controlled Trial Adverse Events Reported in > 5% of Patients and More Commonly in Patients Treated with Pioglitazone + Metformin than in Patients Treated with Placebo + Metformin | ||
|---|---|---|
| % of Patients | ||
| Placebo + Metformin N=160 |
Pioglitazone 30 mg + Metformin N=168 |
|
| Edema |
2.5 |
6 |
| Headache |
1.9 |
6 |
| |
24-Week Non-Controlled Double-Blind Trial Adverse Events Reported in > 5% of Patients and More Commonly in Patients Treated with Pioglitazone 45 mg + Metformin than in Patients Treated with Pioglitazone 30 mg + Metformin
|
|
|
% of Patients
|
||
|
Pioglitazone 30 mg
+ Metformin N=411 |
Pioglitazone 45 mg
+ Metformin N=416 |
|
| Upper Respiratory Tract Infection |
12.4 |
13.5 |
| Edema |
5.8 |
13.9 |
| Headache |
5.4 |
5.8 |
| Weight Increased |
2.9 |
6.7 |
Common Adverse Events: PROactive Trial
| % of Patients | ||
|---|---|---|
| Placebo N=2633 |
Pioglitazone N=2605 |
|
| Hypoglycemia |
18.8 |
27.3 |
| Edema |
15.3 |
26.7 |
| Cardiac Failure |
6.1 |
8.1 |
| Pain in Extremity |
5.7 |
6.4 |
| Back Pain |
5.1 |
5.5 |
| Chest Pain |
5 |
5.1 |
Congestive Heart Failure:
| Number (%) of Patients | ||||
|---|---|---|---|---|
| Placebo-Controlled Trial (16 weeks) |
Non-Controlled Double- Blind Trial (24 weeks) |
|||
| Placebo + Metformin N=160 |
Pioglitazone 30 mg + Metformin N=168 |
Pioglitazone 30 mg + Metformin N=411 |
Pioglitazone 45 mg + Metformin N=416 |
|
| At least one congestive heart failure event |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
| Hospitalized |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
| Patients Treated with Pioglitazone or Placebo Added on to a Sulfonylurea | |||||||
|---|---|---|---|---|---|---|---|
| Number (%) of Patients | |||||||
| Placebo-Controlled Trial (16 weeks) |
Non-Controlled Double- Blind Trial (24 weeks) |
||||||
| Placebo + Sulfonylurea N=187 |
Pioglitazone 15 mg + Sulfonylurea N=184 |
Pioglitazone 30 mg + Sulfonylurea N=189 |
Pioglitazone 30 mg + Sulfonylurea N=351 |
Pioglitazone 45 mg + Sulfonylurea N=351 |
|||
| At least one congestive heart failure event |
2 (1.1%) |
0 |
0 |
1 (0.3%) |
6 (1.7%) |
||
| Hospitalized |
2 (1.1%) |
0 |
0 |
0 |
2 (0.6%) |
||
|
Patients Treated with Pioglitazone or Placebo Added on to Insulin
|
|||||||
| |
Number (%) of Patients
|
||||||
|
Placebo-Controlled Trial
(16 weeks) |
Non-Controlled Double-
Blind Trial (24 weeks) |
||||||
|
Placebo +
Insulin N=187 |
Pioglitazone
15 mg + Insulin N=191 |
Pioglitazone
30 mg + Insulin N=188 |
Pioglitazone
30 mg + Insulin N=345 |
Pioglitazone
45 mg + Insulin N=345 |
|||
| At least one congestive heart failure event |
0 |
2 (1%) |
2 (1.1%) |
3 (0.9%) |
5 (1.4%) |
||
| Hospitalized |
0 |
2 (1%) |
1 (0.5%) |
1 (0.3%) |
3 (0.9%) |
||
|
Patients Treated with Pioglitazone or Placebo Added on to Metformin
|
|||||||
| |
Number (%) of Patients
|
||||||
|
Placebo-Controlled Trial
(16 weeks) |
Non-Controlled Double-
Blind Trial (24 weeks) |
||||||
|
Placebo +
Metformin N=160 |
Pioglitazone
30 mg + Metformin N=168 |
Pioglitazone
30 mg + Metformin N=411 |
Pioglitazone
45 mg + Metformin N=416 |
||||
| At least one congestive heart failure event |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
|||
| Hospitalized |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
|||
| Number (%) of Subjects | ||
|---|---|---|
| Pioglitazone N=262 |
Glyburide N=256 |
|
| Death due to cardiovascular causes (adjudicated) |
5 (1.9%) |
6 (2.3%) |
| Overnight hospitalization for worsening CHF (adjudicated) |
26 (9.9%) |
12 (4.7%) |
| Emergency room visit for CHF (adjudicated) |
4 (1.5%) |
3 (1.2%) |
| Patients experiencing CHF progression during study |
35 (13.4%) |
21 (8.2%) |
| Number (%) of Patients | ||
|---|---|---|
| Placebo N=2633 |
Pioglitazone N=2605 |
|
| At least one hospitalized congestive heart failure event |
108 (4.1%) |
149 (5.7%) |
| Fatal |
22 (0.8%) |
25 (1%) |
| Hospitalized, non-fatal |
86 (3.3%) |
124 (4.7%) |
Cardiovascular Safety:
| Cardiovascular Events | Placebo N=2633 |
Pioglitazone N=2605 |
||
|---|---|---|---|---|
| First Events | Total Events | First Events n (%) |
Total Events | |
| CABG = coronary artery bypass grafting; PCI = percutaneous intervention |
||||
| Any event |
572 (21.7) |
900 |
514 (19.7) |
803 |
| All-cause mortality |
122 (4.6) |
186 |
110 (4.2) |
177 |
| Non-fatal myocardial infarction (MI) |
118 (4.5) |
157 |
105 (4) |
131 |
| Stroke |
96 (3.6) |
119 |
76 (2.9) |
92 |
| Acute coronary syndrome |
63 (2.4) |
78 |
42 (1.6) |
65 |
| Cardiac intervention (CABG/PCI) |
101 (3.8) |
240 |
101 (3.9) |
195 |
| Major leg amputation |
15 (0.6) |
28 |
9 (0.3) |
28 |
| Leg revascularization |
57 (2.2) |
92 |
71 (2.7) |
115 |
Weight Gain:
| Control Group (Placebo) |
Pioglitazone 15 mg |
Pioglitazone 30 mg |
Pioglitazone 45 mg |
||
|---|---|---|---|---|---|
| Median (25th, 75th percentile) |
Median (25th, 75th percentile) |
Median (25th, 75th percentile) |
Median (25th, 75th percentile) |
||
|
Monotherapy
(16 to 26 weeks) |
|
-1.4 (-2.7, 0) N=256 |
0.9 (-0.5, 3.4) N=79 |
1 (-0.9, 3.4) N=188 |
2.6 (0.2, 5.4) N=79 |
|
Combination
Therapy (16 to 24 weeks) |
Sulfonylurea |
-0.5 (-1.8, 0.7) N=187 |
2 (0.2, 3.2) N=183 |
3.1 (1.1, 5.4) N=528 |
4.1 (1.8, 7.3) N=333 |
| Metformin |
-1.4 (-3.2, 0.3) N=160 |
N/A |
0.9 (-1.3, 3.2) N=567 |
1.8 (-0.9, 5) N=407 |
|
| Insulin |
0.2 (-1.4, 1.4) N=182 |
2.3 (0.5, 4.3) N=190 |
3.3 (0.9, 6.3) N=522 |
4.1 (1.4, 6.8) N=338 |
|
| Placebo | Pioglitazone | |
|---|---|---|
| Median (25th, 75th percentile) |
Median (25th, 75th percentile) |
|
| Change from Baseline to Final Visit (kg) |
-0.5 (-3.3, 2) N=2581 |
+3.6 (0, 7.5) N=2560 |
Edema:
| Number (%) of Patients | |||||
|---|---|---|---|---|---|
| Placebo | Pioglitazone 15 mg |
Pioglitazone 30 mg |
Pioglitazone 45 mg |
||
|
Monotherapy (16 to 26 weeks)
|
3 (1.2%) N=259 |
2 (2.5%) N=81 |
13 (4.7%) N=275 |
11 (6.5%) N=169 |
|
|
Combined Therapy
(16 to 24 weeks) |
Sulfonylurea |
4 (2.1%) N=187 |
3 (1.6%) N=184 |
61 (11.3%) N=540 |
81 (23.1%) N=351 |
| Metformin |
4 (2.5%) N=160 |
N/A |
34 (5.9%) N=579 |
58 (13.9%) N=416 |
|
| Insulin |
13 (7%) N=187 |
24 (12.6%) N=191 |
109 (20.5%) N=533 |
90 (26.1%) N=345 |
|
| Number (%) of Patients | |
|---|---|
| Placebo N=2633 |
Pioglitazone N=2605 |
| 419 (15.9%) |
712 (27.3%) |
Hepatic Effects:
Hypoglycemia:
Urinary Bladder Tumors: [see Nonclinical Toxicology (13.1)]
Metformin Hydrochloride
| Adverse Reaction | Metformin Monotherapy (n=141) |
Placebo (n=145) |
|---|---|---|
| % of Patients | ||
| * Reactions that were more common in metformin than placebo-treated patients. |
||
| Diarrhea |
53.2 |
11.7 |
| Nausea/Vomiting |
25.5 |
8.3 |
| Flatulence |
12.1 |
5.5 |
| Asthenia |
9.2 |
5.5 |
| Indigestion |
7.1 |
4.1 |
| Abdominal Discomfort |
6.4 |
4.8 |
| Headache |
5.7 |
4.8 |
6.2 Laboratory Abnormalities
Hematologic Effects:
Vitamin B12 Concentrations: 12 [see Warnings and Precautions (5.14)]
Creatine Phosphokinase:
6.3 Postmarketing Experience
Pioglitazone
- New onset or worsening diabetic macular edema with decreased visual acuity [see Warnings and Precautions (5.8)].
- Fatal and non-fatal hepatic failure [see Warnings and Precautions (5.4)].
[see Boxed Warning and Warnings and Precautions (5.1)]
7 DRUG INTERACTIONS
7.1 Strong CYP2C8 Inhibitors
[see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]
7.2 CYP2C8 Inducers
[see Clinical Pharmacology (12.3)]
7.3 Carbonic Anhydrase Inhibitors
7.4 Cationic Drugs
[see Clinical Pharmacology (12.3)]
7.5 Drugs Affecting Glycemic Control
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pioglitazone Clinical Considerations:
Animal Data: 2[see Nonclinical Toxicology (13.3)]222
Metformin Hydrochloride
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
[see Warnings and Precautions (5.1, 5.2, 5.5, 5.6)].
8.5 Geriatric Use
Pioglitazone
[see Clinical Pharmacology (12.3)]
Metformin Hydrochloride
[see Contraindications (4) Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)][see Warnings and Precautions (5.2) and Dosage and Administration (2)]
10 OVERDOSAGE
Pioglitazone
Metformin Hydrochloride
[see Warnings and Precautions (5.2)]
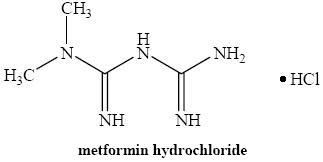
11 DESCRIPTION
in vivo

192023N,N
N,N4115
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pioglitazone
Metformin Hydrochloride
see Warnings and Precautions (5.7)
12.2 Pharmacodynamics
Pioglitazone
[see Clinical Studies (14)]
[see Warnings and Precautions (5.15) and Adverse Reactions (6.1)]
| Placebo | Pioglitazone 15 mg Once Daily |
Pioglitazone 30 mg Once Daily |
Pioglitazone 45 mg Once Daily |
|
|---|---|---|---|---|
| * Adjusted for baseline, pooled center, and pooled center by treatment interaction † p < 0.05 versus placebo |
||||
|
Triglycerides (mg/dL)
|
N=79 |
N=79 |
N=84 |
N=77 |
| Baseline (mean) |
263 |
284 |
261 |
260 |
| Percent change from baseline (adjusted mean*) |
4.8% |
-9%†
|
-9.6%†
|
-9.3%†
|
|
HDL Cholesterol (mg/dL)
|
N=79 |
N=79 |
N=83 |
N=77 |
| Baseline (mean) |
42 |
40 |
41 |
41 |
| Percent change from baseline (adjusted mean*) |
8.1% |
14.1%†
|
12.2% |
19.1%†
|
|
LDL Cholesterol (mg/dL)
|
N=65 |
N=63 |
N=74 |
N=62 |
| Baseline (mean) |
139 |
132 |
136 |
127 |
| Percent change from baseline (adjusted mean*) |
4.8% |
7.2% |
5.2% |
6% |
|
Total Cholesterol (mg/dL)
|
N=79 |
N=79 |
N=84 |
N=77 |
| Baseline (mean) |
225 |
220 |
223 |
214 |
| Percent change from baseline (adjusted mean*) |
4.4% |
4.6% |
3.3% |
6.4% |
12.3 Pharmacokinetics
Absorption
Pioglitazone Hydrochloride and Metformin Hydrochloride
max
Pioglitazone
maxmin
max
Metformin Hydrochloride
Distribution
Pioglitazone
Metformin Hydrochloride
Metabolism
Pioglitazone
In vitro In vivo[see Dosage and Administration (2.3) and Drug Interactions (7.1)]
Metformin Hydrochloride
Excretion and Elimination
Pioglitazone
Metformin Hydrochloride
Specific Populations
Renal Impairment
Pioglitazone
Metformin Hydrochloride
[see Contraindications (4) and Warnings and Precautions (5.2)]
Hepatic Impairment
Pioglitazone
[see Warnings and Precautions (5.4)]
Metformin Hydrochloride
[see Warnings and Precautions (5.4)]
Geriatric Patients
Pioglitazone
Metformin Hydrochloride
max
[see Warnings and Precautions (5.2)]
Pediatrics
Pioglitazone
[see Use in Specific Populations (8.4)]
Metformin Hydrochloride
max
Gender
Pioglitazone
max
Metformin Hydrochloride
Ethnicity
Pioglitazone
Metformin Hydrochloride
Drug-Drug Interactions
Pioglitazone
| Co-administered Drug | ||||||
|---|---|---|---|---|---|---|
| Pioglitazone Dosage Regimen (mg)* |
Name and Dose Regimens | Change in AUC† |
Change in Cmax † |
|||
| * Daily for 7 days unless otherwise noted † % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively. ‡ Pioglitazone had no clinically significant effect on prothrombin time |
||||||
| 45 mg (N = 12) |
Warfarin‡ |
|||||
| Daily loading then maintenance doses based PT and INR values Quick's Value = 35 ± 5% |
R-Warfarin |
↓3% |
R-Warfarin |
↓2% |
||
| S-Warfarin |
↓1% |
S-Warfarin |
↑1% |
|||
| 45 mg (N = 12) |
Digoxin
|
|||||
| 0.2 mg twice daily (loading dose) then 0.25 mg daily (maintenance dose, 7 days) |
↑15% |
↑17% |
||||
| 45 mg daily for 21 days (N = 35) |
Oral Contraceptive
|
|||||
| [Ethinyl Estradiol (EE) 0.035 mg plus Norethindrone (NE) 1 mg] for 21 days |
EE |
↓11% |
EE |
↓13% |
||
| NE |
↑3% |
NE |
↓7% |
|||
| 45 mg (N = 23) |
Fexofenadine
|
|||||
| 60 mg twice daily for 7 days |
↑30% |
↑37% |
||||
| 45 mg (N = 14) |
Glipizide
|
|||||
| 5 mg daily for 7 days |
↓3% |
↓8% |
||||
| 45 mg daily for 8 days (N = 16) |
Metformin
|
|||||
| 1000 mg single dose on Day 8 |
↓3% |
↓5% |
||||
| 45 mg (N = 21) |
Midazolam
|
|||||
| 7.5 mg single dose on Day 15 |
↓26% |
↓26% |
||||
| 45 mg (N = 24) |
Ranitidine
|
|||||
| 150 mg twice daily for 7 days |
↑1% |
↓1% |
||||
| 45 mg daily for 4 days (N = 24) |
Nifedipine ER
|
|||||
| 30 mg daily for 4 days |
↓13% |
↓17% |
||||
| 45 mg (N = 25) |
Atorvastatin Ca
|
|||||
| 80 mg daily for 7 days |
↓14% |
↓23% |
||||
| 45 mg (N = 22) |
Theophylline
|
|||||
| 400 mg twice daily for 7 days |
↑2% |
↑5% |
||||
| Coadministered Drug and Dosage Regimen |
Pioglitazone | ||
|---|---|---|---|
| Dose Regimen (mg)* |
Change in AUC† |
Change in Cmax† |
|
| * Daily for 7 days unless otherwise noted † Mean ratio (with/without coadministered drug and no change = 1-fold) % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively. ‡ The half-life of pioglitazone increased from 6.5 h to 15.1 h in the presence of gemfibrozil [see Dosage and Administration (2.3) and Drug Interactions (7.1)] |
|||
| Gemfibrozil 600 mg twice daily for 2 days (N = 12) |
30 mg single dose |
↑ 3.4-fold‡
|
↑ 6% |
| Ketoconazole 200 mg twice daily for 7 days (N = 28) |
45 mg |
↑ 34% |
↑ 14% |
| Rifampin 600 mg daily for 5 days (N = 10) |
30 mg single dose |
↓ 54% |
↓ 5% |
| Fexofenadine 60 mg twice daily for 7 days (N = 23) |
45 mg |
↑ 1% |
0% |
| Ranitidine 150 mg twice daily for 4 days (N = 23) |
45 mg |
↓ 13% |
↓ 16% |
| Nifedipine ER 30 mg daily for 7 days (N = 23) |
45 mg |
↑ 5% |
↑ 4% |
| Atorvastatin Ca 80 mg daily for 7 days (N = 24) |
45 mg |
↓ 24% |
↓ 31% |
| Theophylline 400 mg twice daily for 7 days (N = 22) |
45 mg |
↓ 4% |
↓ 2% |
Metformin hydrochloride
| Coadministered Drug |
Dose of Coadministered Drug* |
Dose of Metformin* |
Geometric Mean Ratio (ratio with/without coadministered drug) No effect = 1 |
|||
|---|---|---|---|---|---|---|
| AUC† | Cmax | |||||
| * All metformin and coadministered drugs were given as single doses † AUC = AUC0–∞ ‡ Ratio of arithmetic means § Metformin hydrochloride extended-release tablets, 500 mg ¶ At steady state with topiramate 100 mg every 12 hours and metformin 500 mg every 12 hours; AUC = AUC0-12h |
||||||
| No dosing adjustments required for the following: |
||||||
| Glyburide |
5 mg |
500 mg§
|
0.98‡
|
0.99‡
|
||
| Furosemide |
40 mg |
850 mg |
1.09‡
|
1.22‡
|
||
| Nifedipine |
10 mg |
850 mg |
1.16 |
1.21 |
||
| Propranolol |
40 mg |
850 mg |
0.9 |
0.94 |
||
| Ibuprofen |
400 mg |
850 mg |
1.05‡
|
1.07‡
|
||
| Cationic drugs eliminated by renal tubular secretion may reduce metformin elimination: use with caution [see Warnings and Precautions (5) and Drug Interactions (7)]. |
||||||
| Cimetidine |
400 mg |
850 mg |
1.4 |
1.61 |
||
| Carbonic anhydrase inhibitors may cause metabolic acidosis: use with caution [see Warnings and Precautions (5) and Drug Interactions (7)]. |
||||||
| Topiramate |
100 mg¶
|
500 mg¶
|
1.25¶
|
1.17 |
||
| Coadministered Drug |
Dose of Coadministered Drug* |
Dose of Metformin* |
Geometric Mean Ratio (ratio with/without coadministered drug) No effect = 1 |
|
|---|---|---|---|---|
| AUC† | Cmax | |||
| * All metformin and coadministered drugs were given as single doses † AUC = AUC0–∞ ‡ Ratio of arithmetic means, p-value of difference <0.05 § AUC0-24 hr reported ¶ Ratio of arithmetic means |
||||
| No dosing adjustments required for the following: |
||||
| Glyburide |
5 mg |
500 mg§
|
0.78‡
|
0.63‡
|
| Furosemide |
40 mg |
850 mg |
0.87‡
|
0.69‡
|
| Nifedipine |
10 mg |
850 mg |
1.1§
|
1.08 |
| Propranolol |
40 mg |
850 mg |
1.01§
|
0.94 |
| Ibuprofen |
400 mg |
850 mg |
0.97¶
|
1.01¶
|
| Cimetidine |
400 mg |
850 mg |
0.95§
|
1.01 |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Pioglitazone Hydrochloride and Metformin Hydrochloride
Pioglitazone
222
in vitroin vivo
2
Metformin Hydrochloride
in vitro S. typhimuriumin vivo
13.2 Animal Toxicology and/or Pharmacology
2222
13.3 Reproductive and Developmental Toxicology
2222
14 CLINICAL STUDIES
14.2 Patients Previously Treated with Metformin
[see Clinical Pharmacology (12.3)]
(see Table 22)
| Placebo + Metformin |
Pioglitazone 30 mg + Metformin |
|
|---|---|---|
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction † p ≤ 0.05 vs. placebo + metformin |
||
|
Total Population
|
||
|
HbA1c (%)
|
N=153 |
N=161 |
| Baseline (mean) |
9.8 |
9.9 |
| Change from baseline (adjusted mean*) |
0.2 |
-0.6 |
| Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval |
-0.8†
(-1.2, -0.5) |
|
|
Fasting Plasma Glucose (mg/dL)
|
N=157 |
N=165 |
| Baseline (mean) |
260 |
254 |
| Change from baseline (adjusted mean*) |
-5 |
-43 |
| Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval |
-38†
(-49, -26) |
|
(see Table 23)
| Pioglitazone 30 mg + Metformin |
Pioglitazone 45 mg + Metformin |
|
|---|---|---|
| 95% CI = 95% confidence interval *Adjusted for baseline, pooled center, and pooled center by treatment interaction † p ≤ 0.05 vs. 30 mg daily pioglitazone + metformin |
||
|
Total Population
|
||
|
HbA1c (%)
|
N=400 |
N=398 |
| Baseline (mean) |
9.9 |
9.8 |
| Change from baseline (adjusted mean*) |
-0.8 |
-1 |
| Difference from 30 mg daily Pioglitazone + Metformin (adjusted mean*) (95% CI) |
-0.2 (-0.5, 0.1) |
|
|
Fasting Plasma Glucose (mg/dL)
|
N=398 |
N=399 |
| Baseline (mean) |
233 |
232 |
| Change from baseline (adjusted mean *) |
-38 |
-51 |
| Difference from 30 mg daily Pioglitazone + Metformin (adjusted mean*) (95% CI) |
-12†
(-21, -4) |
|
16 HOW SUPPLIED/STORAGE AND HANDLING
Pioglitazone Hydrochloride and Metformin Hydrochloride Tablets, 15 mg/500 mg
Pioglitazone Hydrochloride and Metformin Hydrochloride Tablets, 15 mg/850 mg
Storage: Store at o
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
17.1 Instructions
- It is important to instruct patients to adhere to dietary instructions and to have blood glucose and glycosylated hemoglobin tested regularly. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be reminded to seek medical advice promptly.
- Tell patients to promptly report any sign of macroscopic hematuria or other symptoms such as dysuria or urinary urgency that develop or increase during treatment as these may be due to bladder cancer.
- Explain to patients the risks of lactic acidosis, its symptoms and conditions that predispose to its development, as noted in the Warnings and Precautions (5.2) section. Advise patients to discontinue pioglitazone hydrochloride and metformin hydrochloride immediately and to promptly notify their healthcare professional if unexplained hyperventilation, myalgia, gastrointestinal symptoms, malaise, unusual somnolence or other nonspecific symptoms occur.
- Counsel patients against excessive alcohol intake while receiving pioglitazone hydrochloride and metformin hydrochloride.
- Inform patients to immediately report symptoms of an unusually rapid increase in weight or edema, shortness of breath or other symptoms of heart failure while receiving pioglitazone hydrochloride and metformin hydrochloride.
- Tell patients to promptly stop taking pioglitazone hydrochloride and metformin hydrochloride and seek immediate medical advice if there is unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine as these symptoms may be due to hepatotoxicity.
- Inform patients about the importance of regular testing of renal function and hematologic parameters when receiving treatment with pioglitazone hydrochloride and metformin hydrochloride.
- Therapy with a thiazolidinedione, which is the active pioglitazone component of the pioglitazone hydrochloride and metformin hydrochloride tablet, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking pioglitazone hydrochloride and metformin hydrochloride. Recommend adequate contraception for all pre-menopausal women who are prescribed pioglitazone hydrochloride and metformin hydrochloride.
- Patients should be advised to notify their health practitioner or call the Poison Control Center immediately in case of pioglitazone hydrochloride and metformin hydrochloride overdose.
- Combination antihyperglycemic therapy may cause hypoglycemia. When initiating pioglitazone hydrochloride and metformin hydrochloride, the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and their family members.
- Patients should be told to take pioglitazone hydrochloride and metformin hydrochloride as prescribed and instructed that any change in dosing should only be done if directed by their physician. If a dose is missed on one day, the dose should not be doubled the following day.
17.2 FDA-Approved Medication Guide
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
MEDICATION GUIDE
Pioglitazone Hydrochloride and Metformin Hydrochloride Tablets
What is the most important information I should know about pioglitazone hydrochloride and metformin hydrochloride tablets?
Pioglitazone hydrochloride and metformin hydrochloride tablets can cause serious side effects, including:
-
New or worse heart failure. Pioglitazone, one of the medicines in pioglitazone hydrochloride and metformin hydrochloride tablets, can cause your body to keep extra fluid (fluid retention), which leads to swelling (edema) and weight gain. Extra body fluid can make some heart problems worse or lead to heart failure. Heart failure means your heart does not pump blood well enough.
- Do not take pioglitazone hydrochloride and metformin hydrochloride tablets if you have severe heart failure
- If you have heart failure with symptoms (such as shortness of breath or swelling), even if these symptoms are not severe, pioglitazone hydrochloride and metformin hydrochloride tablets may not be right for you.
Call your doctor right away if you have any of the following:
- swelling or fluid retention, especially in the ankles or legs
- shortness of breath or trouble breathing, especially when you lie down
- an unusually fast increase in weight
- unusual tiredness
- Lactic Acidosis. Metformin, one of the medicines in pioglitazone hydrochloride and metformin hydrochloride tablets, can cause a rare but serious condition called lactic acidosis (a buildup of an acid in the blood) that can cause death. Lactic acidosis is a medical emergency and must be treated in the hospital.
Call your doctor right away if you have any of the following symptoms which could be signs of lactic acidosis:
- you feel weak or tired
- you have unusual (not normal) muscle pain
- you have stomach pains, nausea or vomiting
- you have trouble breathing
- you feel dizzy or lightheaded
- you have a slow or irregular heartbeat
- have kidney problems or your kidneys are affected by certain x-ray tests that use injectable dye. People whose kidneys are not working properly should not take pioglitazone hydrochloride and metformin hydrochloride tablets
- have liver problems
- drink alcohol very often, or drink a lot of alcohol in short-term "binge" drinking
- get dehydrated (lose a large amount of body fluids). This can happen if you are sick with a fever, vomiting, or diarrhea. Dehydration can also happen when you sweat a lot with activity or exercise and do not drink enough fluids
- have surgery
- have a heart attack, severe infection, or stroke
- are 80 years of age or older and have not had your kidneys tested
“What are the possible side effects of pioglitazone hydrochloride and metformin hydrochloride tablets?”
What are pioglitazone hydrochloride and metformin hydrochloride tablets?
Who should not take pioglitazone hydrochloride and metformin hydrochloride tablets?
“What is the most important information I should know about pioglitazone hydrochloride and metformin hydrochloride tablets?”
Do not take pioglitazone hydrochloride and metformin hydrochloride tablets if you:
- have severe heart failure
- are allergic to pioglitazone, metformin or any of the ingredients in pioglitazone hydrochloride and metformin hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in pioglitazone hydrochloride and metformin hydrochloride tablets
- have kidneys which are not working properly
- have a condition called metabolic acidosis, including diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin
What should I tell my doctor before taking pioglitazone hydrochloride and metformin hydrochloride tablets?
- have heart failure
- have kidney problems
- are going to have dye injected into a vein for an x-ray, CAT scan, heart study, or other type of scanning
- will be undergoing a surgical procedure
- drink a lot of alcohol (all the time or short binge drinking)
- have type 1 (“juvenile”) diabetes or had diabetic ketoacidosis
- have a type of diabetic eye disease that causes swelling in the back of the eye (macular edema)
- have liver problems
- are pregnant or plan to become pregnant. It is not known if pioglitazone hydrochloride and metformin hydrochloride tablets will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant about the best way to control your blood glucose levels while pregnant
- are a premenopausal woman (before the “change of life”), who does not have periods regularly or at all. Pioglitazone hydrochloride and metformin hydrochloride tablets may increase your chance of becoming pregnant. Talk to your doctor about birth control choices while taking pioglitazone hydrochloride and metformin hydrochloride tablets. Tell your doctor right away if you become pregnant while taking pioglitazone hydrochloride and metformin hydrochloride tablets
- are breast-feeding or plan to breast-feed. It is not known if pioglitazone hydrochloride and metformin hydrochloride passes into your breast milk. You and your doctor should decide if you will take pioglitazone hydrochloride and metformin hydrochloride tablets or breastfeed. You should not do both. Talk to your doctor about the best way to control your blood glucose levels while breastfeeding
Tell your doctor about all the medicines you take
How should I take pioglitazone hydrochloride and metformin hydrochloride tablets?
- Take pioglitazone hydrochloride and metformin hydrochloride tablets exactly as your doctor tells you to take them.
- Your doctor may need to change your dose of pioglitazone hydrochloride and metformin hydrochloride tablets. Do not change your pioglitazone hydrochloride and metformin hydrochloride tablets dose unless your doctor tells you to
- Pioglitazone hydrochloride and metformin hydrochloride tablets may be prescribed alone or with other diabetes medicines. This will depend on how well your blood sugar is controlled
- Take pioglitazone hydrochloride and metformin hydrochloride tablets with meals to lower your chance of an upset stomach
- If you miss a dose of pioglitazone hydrochloride and metformin hydrochloride tablets, take your next dose as prescribed unless your doctor tells you differently. Do not take two doses at one time the next day
- If you take too much pioglitazone hydrochloride and metformin hydrochloride, call your doctor or go to the nearest hospital emergency room right away
- If your body is under stress such as from a fever, infection, accident, or surgery, the dose of your diabetes medicines may need to be changed. Call your doctor right away
- Stay on your diet and exercise programs and test your blood sugar regularly while taking pioglitazone hydrochloride and metformin hydrochloride tablets
- Your doctor should do certain blood tests before you start and while you take pioglitazone hydrochloride and metformin hydrochloride tablets
- Your doctor should also do hemoglobin A1C testing to check how well your blood sugar is controlled with pioglitazone hydrochloride and metformin hydrochloride tablets
- Your doctor should check your eyes regularly while you take pioglitazone hydrochloride and metformin hydrochloride tablets
What are the possible side effects of pioglitazone hydrochloride and metformin hydrochloride tablets?
Pioglitazone hydrochloride and metformin hydrochloride tablets may cause serious side effects including:
- See “What is the most important information I should know about pioglitazone hydrochloride and metformin hydrochloride tablets?”
-
liver problems. Call your doctor right away if you have:
- nausea or vomiting
- stomach pain
- unusual or unexplained tiredness
- loss of appetite
- dark urine
- yellowing of your skin or the whites of your eyes
- broken bones (fractures). Usually in the hand, upper arm, or foot in women. Talk to your doctor for advice on how to keep your bones healthy
-
bladder cancer. There may be an increased chance of having bladder cancer when you take pioglitazone hydrochloride and metformin hydrochloride tablets. You should not take pioglitazone hydrochloride and metformin hydrochloride tablets if you are receiving treatment for bladder cancer. Tell your doctor right away if you have any of the following symptoms of bladder cancer:
- blood or a red color in your urine
- an increased need to urinate
- pain while you urinate
- low blood sugar (hypoglycemia). This can happen if you skip meals, if you also use another medicine that lowers blood sugar, or if you have certain medical problems. Lightheadedness, dizziness, shakiness, or hunger may happen if your blood sugar is too low. Call your doctor if low blood sugar levels are a problem for you
- diabetic eye disease with swelling in the back of the eye (macular edema). Tell your doctor right away if you have any changes in your vision. Your doctor should check your eyes regularly
- release of an egg from an ovary in a woman (ovulation) leading to pregnancy. Ovulation may happen when premenopausal women who do not have regular monthly periods take pioglitazone hydrochloride and metformin hydrochloride tablets. This can increase your chance of getting pregnant.
- low red blood cell count (anemia).
- cold-like symptoms (upper respiratory tract infection)
- swelling (edema)
- diarrhea
- headache
- increased weight
How should I store pioglitazone hydrochloride and metformin hydrochloride tablets?
- Store pioglitazone hydrochloride and metformin hydrochloride tablets at 20o to 25°C (68o to 77°F). Keep pioglitazone hydrochloride and metformin hydrochloride tablets in the original container and protect from light.
- Keep the pioglitazone hydrochloride and metformin hydrochloride tablets bottle tightly closed and protect from getting wet (away from moisture and humidity).
General information about the safe and effective use of pioglitazone hydrochloride and metformin hydrochloride tablets
What are the ingredients in pioglitazone hydrochloride and metformin hydrochloride tablets?
Active Ingredients
Inactive Ingredients:
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg/500 mg (60 Tablet Bottle)
NDC 65862-525-60
Pioglitazone Hydrochloride
and Metformin Hydrochloride Tablets
15 mg*/500 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx only 60 Tablets
AUROBINDO
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 15 mg/850 mg (60 Tablet Bottle)
NDC 65862-526-60
Pioglitazone Hydrochloride
and Metformin Hydrochloride Tablets
15 mg*/850 mg
PHARMACIST: Dispense the Medication Guide provided separately to each patient.
Rx only 60 Tablets
AUROBINDO
Pioglitazone Hydrochloride and Metformin HydrochloridePioglitazone Hydrochloride and Metformin Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pioglitazone Hydrochloride and Metformin HydrochloridePioglitazone Hydrochloride and Metformin Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||