Pigment
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient: Hydroquinone (2%)
Purpose
Purpose: Skin Lightener
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Uses
Uses: For gradual fading of discolorations of the skin such as freckles, age and liver spots, pigment in the skin that may occur from pregnancy or the use of oral contraceptives.
Directions: Adults: Apply a small amount as a thin layer on the affected area twice daily or use as directed by a physician. If no improvement is seen after 3 months of treatment, use of this product sould be discontinued. Lightening effect of this product may not be noticeable when used on very dark skin. Sun exposure should be limited by using a sunscreen agent or protective clothing when using or after using this product in order to prevent darkening from reoccurring.
Inactive Ingredients: Hamamelis Virginiana (Witch Hazel) Water, Alcohol Denat., Water/Aqua/Eau, Propylene Glycol, Lactic Acid, Azelaic Acid, Kojic Acid, Citric Acid, Hydroxypropyl Methylcellulose.
Principal Display Panel:
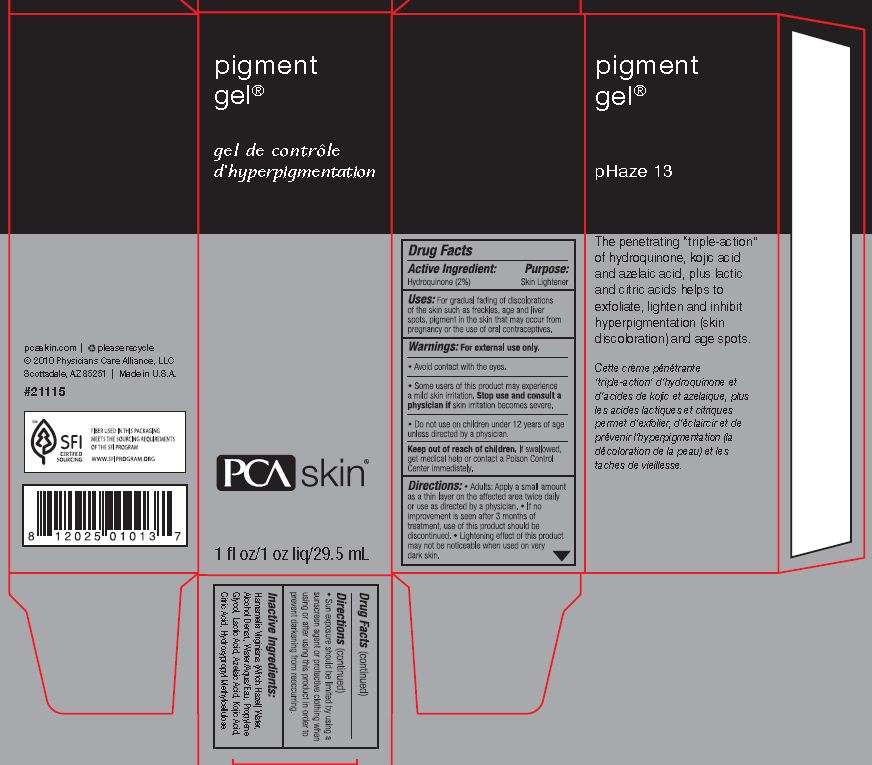
PigmentHydroquinone GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||