Phenylephrine
Phenylephrine Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Phenylephrine Uses
- Warnings
- Directions
- Phenylephrine Other information
- Inactive ingredients
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Phenylephrine hydrochloride 10 mg
Purpose
Nasal decongestant
Keep Out of Reach of Children
In case of overdose, get medical help or contact a Poison Control Center right away.
Phenylephrine Uses
° temporarily relieves sinus congestion and pressure
° temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
Warnings
Do not use if you are now taking a prescription monoamine exidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have:
° heart disease ° high blood pressure ° diabetes ° trouble urinating due to an enlarge prostate gland ° thyroid disease
When using this product, do not use more than directed.
Stop use and ask a doctor if:
° you get nervous, dizzy or sleepless
° symptoms do not improve within 7 days or are accompanied by fever
If pregnant or breast-feeding, ask a health professional before use.
Directions
° take every 4 hours
° do not take more than 6 doses in 24 hours
° adults and children 12 years of age and over: 1 tablet
° children under 12 years of age: ask a doctor
Phenylephrine Other information
Store at room temperature (59°-86°F)
Inactive ingredients
carnauba wax. dibasic calcium phosphate, FD&C red no. 40, lecithin, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, talc, titanium dioxide
Package/Label Principal Display Panel
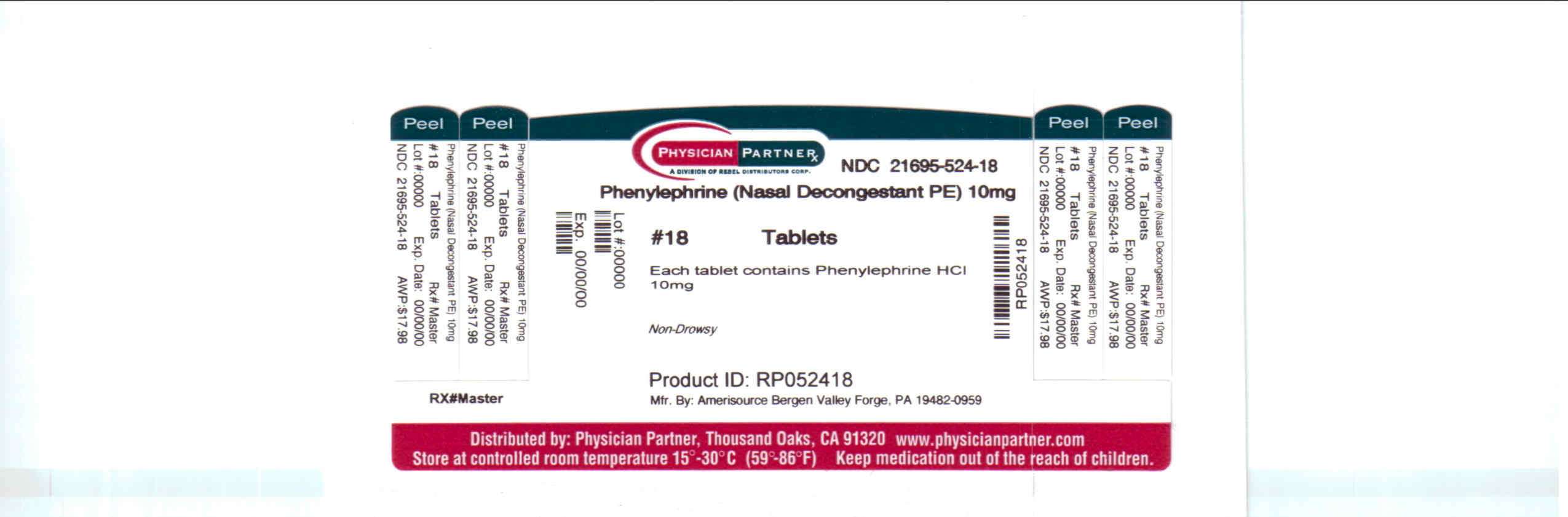
PhenylephrinePhenylephrine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||