Phenylephrine Hydrochloride
Phenylephrine HCl Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Phenylephrine Hydrochloride Uses
- Warnings
- Directions
- Phenylephrine Hydrochloride Other information
- Inactive ingredients
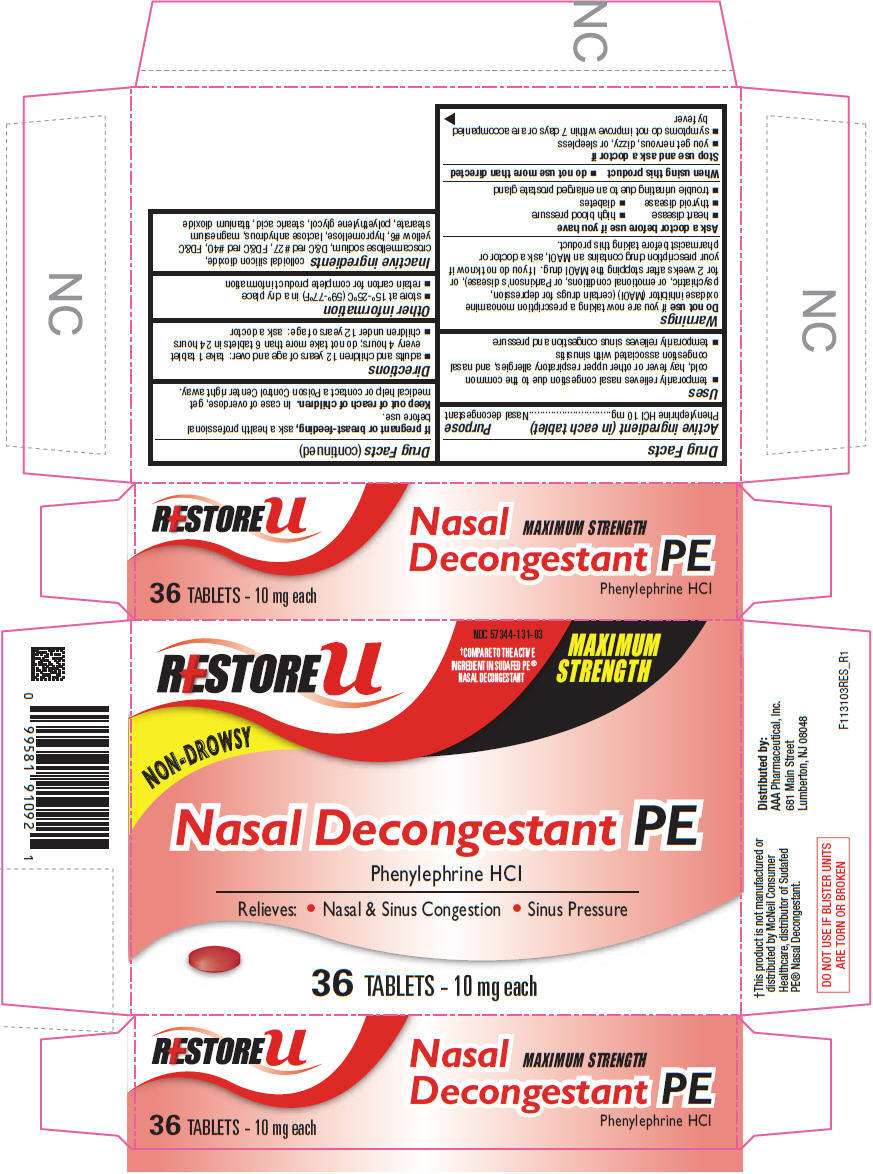
- PRINCIPAL DISPLAY PANEL - 36 Tablet Blister Pack Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient (in each tablet)
Phenylephrine HCl 10 mg
Purpose
Nasal decongestant
Phenylephrine Hydrochloride Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies, and nasal congestion associated with sinusitis
- temporarily relieves sinus congestion and pressure
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
Stop use and ask a doctor if
- you get nervous, dizzy, or sleepless
- symptoms do not improve within 7 days or are accompanied by fever
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and over: take 1 tablet every 4 hours; do not take more than 6 tablets in 24 hours
- children under 12 years of age: ask a doctor
Phenylephrine Hydrochloride Other information
- store at 15°-25°C (59°-77°F) in a dry place
- retain carton for complete product information
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, D&C red #27, FD&C red #40, FD&C yellow #6, hypromellose, lactose anhydrous, magnesium stearate, polyethylene glycol, stearic acid, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
PRINCIPAL DISPLAY PANEL - 36 Tablet Blister Pack Carton
RESTORE u
NDC 57344-131-03
†COMPARE TO THE ACTIVE
INGREDIENT IN SUDAFED PE®
NASAL DECONGESTANT
MAXIMUM
STRENGTH
NON-DROWSY
Nasal Decongestant PE
Phenylephrine HCl
Relieves: • Nasal & Sinus Congestion • Sinus Pressure
36 TABLETS - 10 mg each
Phenylephrine HydrochloridePHENYLEPHRINE HYDROCHLORIDE TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||