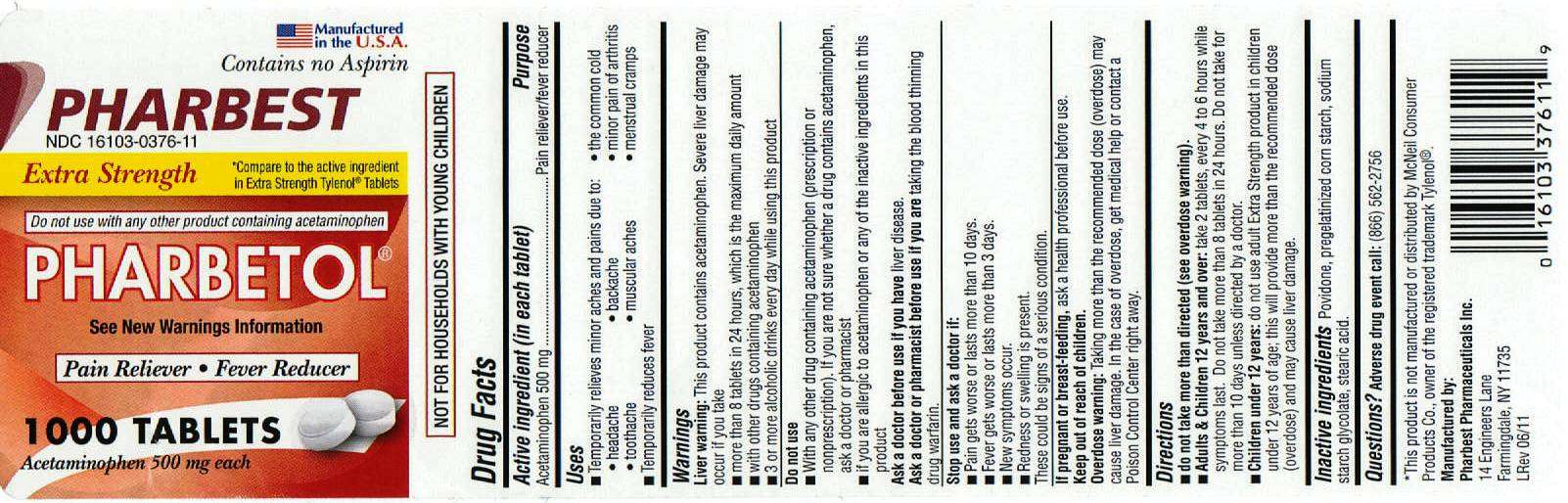
Pharbest Pharmaceuticals, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Purpose
Pharbetol Uses
- headache
- backache
- minor pain of arthritis
- toothache
- muscular aches
- menstrual cramps
Warnings
Liver warning:
- more than 8 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- With any other drug containing
acetaminophen (prescription or nonprescription). If you are not sure
whether a drug contains acetaminophen, ask a doctor or pharmacist
- if you are allergic to acetaminophen or any of
the inactive ingredients in this product
Ask a doctor before use if you have
Ask a doctor or pharmacist before use if you are
Stop use and ask a doctor if:
- Pain gets worse or lasts more than 10 days.
- Fever gets worse or lasts more than 3 days.
- New symptoms occur.
- Redness or swelling is present.
If pregnant or breast-feeding,
Keep out of reach of children.
Overdose warning:
Directions
-
do not take more than directed (see overdose warning).
-
Adult and Children 12 years and over: take 2 tablets, every 4 to 6 hours while symptoms last. Do not take more than 8 tablets in 24 hours. Do not take for more than 10 days unless directed by a doctor.
-
Children under 12 years: do not use adult extra strength product in children under 12 years of age; this will provide more than recommended dose (overdose) and may cause liver damage.
Inactive ingredients
Questions?
Pharbetol
Acetaminophen TABLET
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:16103-376 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
ACETAMINOPHEN ACETAMINOPHEN |
|
500 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
white |
12 mm |
PH044 |
ROUND |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
|
50 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
|
100 in 1 BOTTLE, PLASTIC |
|
|
|
3 |
NDC:16103-376-11 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part343 |
2006-01-10 |
|
|
