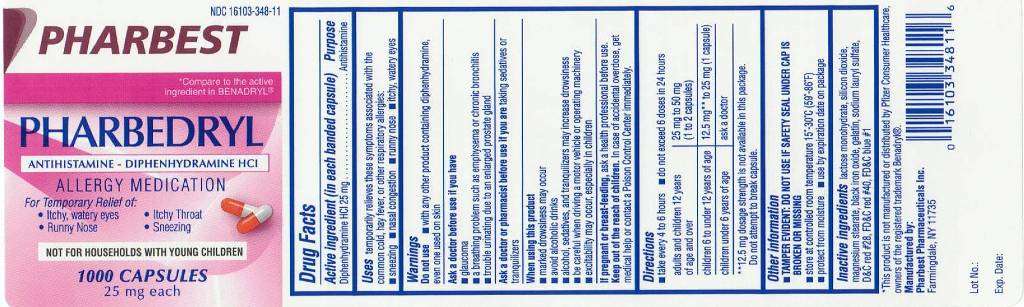
Pharbedryl
Pharbest Pharmaceuticals, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient(in each banded capsule)
- Pharbedryl Uses:
- Warnings:
- Do not use
- Ask a doctor or pharmacist before use
- When using this product
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions:
- Pharbedryl Other information:
- Inactive ingredients: Black Iron Oxide, D & C Red #28, FD & C Blue #1, FD & C Red #40, Gelatin, Lactose Monohydrate, Magnesium Stearate, Silicon Dioxide, Sodium Lauryl Sulfate
- Questions? Adverse drug event call: (866)562-2756
FULL PRESCRIBING INFORMATION
Active ingredient(in each banded capsule)
Diphenhydramine HCL 25 mg
Purpose
Antihistamine
Uses:
- Temporarily relieves these symptoms associated with the common cold, hay fever, or other respiratory allergies:
Warnings:
Do not use
- With any other product containing diphenhydramine, even one used on skin.
Ask a doctor or pharmacist before use
- Trouble urinating due to an enlarged prostate gland
- A breathing problem such as emphysema or chronic bronchitis
- Glaucoma
Ask a doctor or pharmacist before use of you are
When using this product
- Avoid alcoholic drinks
- Marked drowsiness may occur
- Excitability may occur, especially in children
- Alcohol, sedatives and tranquilizers may increase drowsiness
- Be careful when driving a motor vehicle or operating machinery
If pregnant or breast-feeding,
Keep out of reach of children.
Directions:
- Take every 4-6 hours
- Do not exceed 6 doses in 24 hours.
| Adults and children 12 years of age or over |
25 mg to 50 mg (1 to 2 capsules) |
| Children 6 to under 12 years of age |
12.5 mg** to 25 mg (1 capsule) |
| Children under 6 years |
ask a doctor |
Other information:
-
Tamper Evident: Do not use if safety seal under cap is broken or missing
- Store at controlled room temperature 15°-30°C (59°-86°F)
- Protect from moisture
- Use by expiration date on package
Inactive ingredients: Black Iron Oxide, D & C Red #28, FD & C Blue #1, FD & C Red #40, Gelatin, Lactose Monohydrate, Magnesium Stearate, Silicon Dioxide, Sodium Lauryl Sulfate
Questions? Adverse drug event call: (866)562-2756
Enter section text here
PharbedrylDiphenhydramine HCl CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!