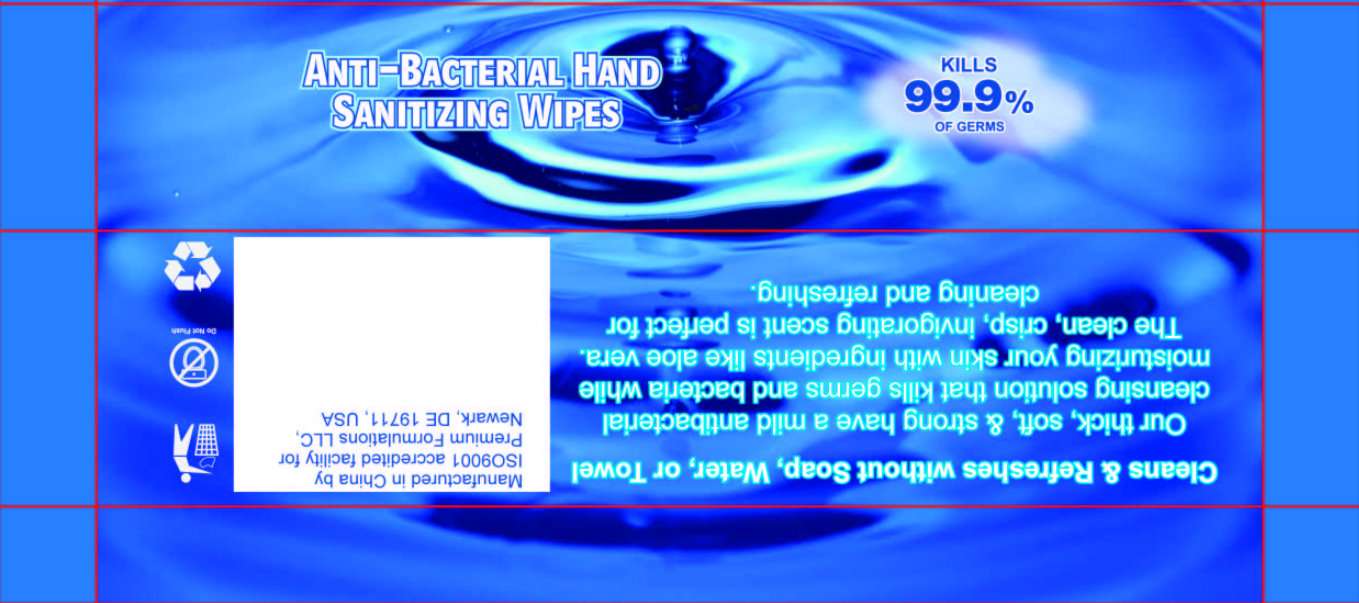
PF ANTI-BACTERIAL HAND SANITIZING WIPES
Premium Formulations LLC
AMERICAN HYGIENICS CORPORATION
PF Anti-Bacterial Hand Sanitizing Wipes
FULL PRESCRIBING INFORMATION
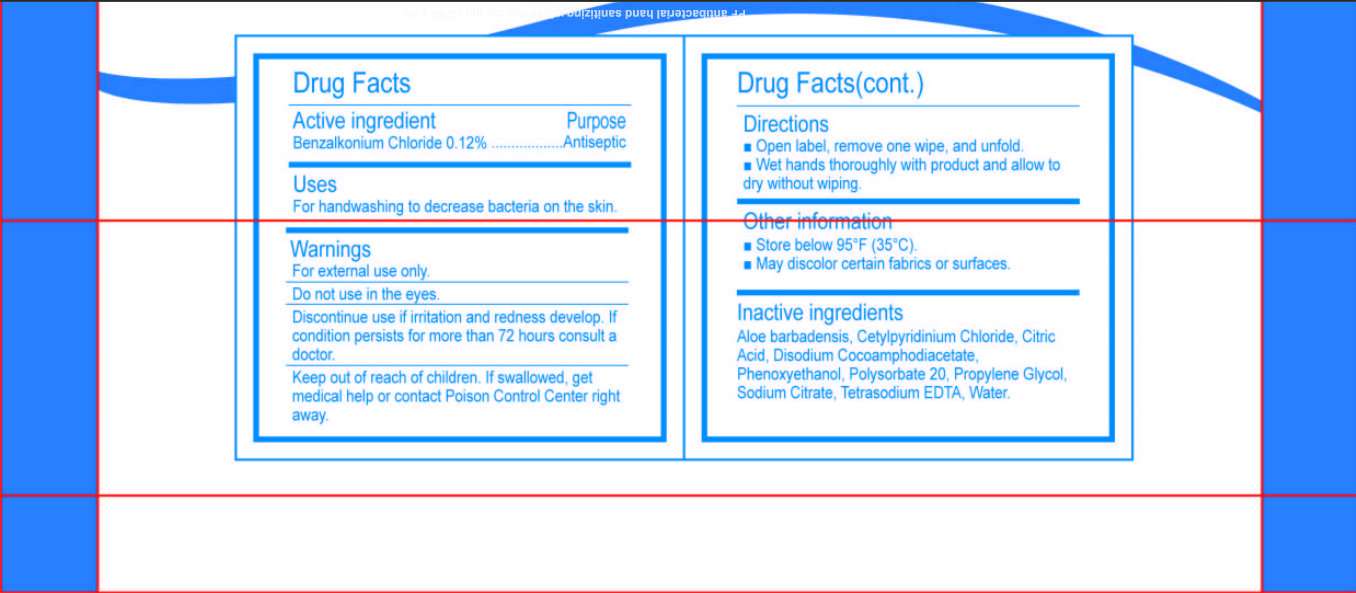
Active ingredient
Active Ingredient
Benzalkonium Chloride 0.12 Percent
Purpose
Uses
Uses
For hand washing to decrease bacteria on the skin.
Warnings
For external use only.
Do not use in the eyes.
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact Poison Control Center right away.
Open label, remove one wipe, and unfold.
Wet hands thoroughly with product and allow to dry without wiping.
Other information
Store below 95 degree Fahrenheit (35 degree Celsius)
May discolor certain fabrics or surfaces.
Inactive ingredients
Aloe barbadensis, Cetylpyridinium Chloride, Citric Acid, Disodium Cocoamphodiacetate, Phenoxyethanol, Polysorbate 20, Propylene Glycol, Sodium Citrate, Tetrasodium EDTA, Water.
PF 25 Wipes
Premium Formulations
ANTI-BACTERIAL HAND SANITIZING WIPES
KILLS 99.9 percent OF GERMS

PF ANTI-BACTERIAL HAND SANITIZING WIPESBENZALKONIUM CHLORIDE LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||