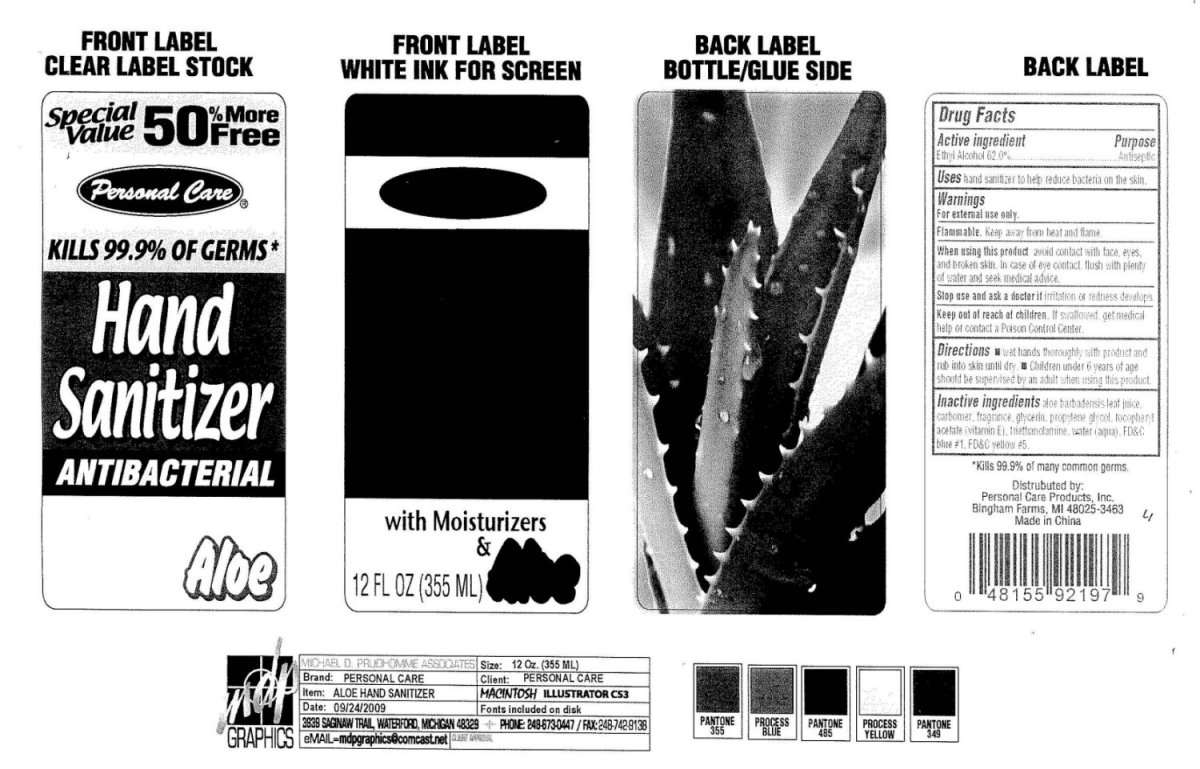
Personal Care Hand Sanitizer Aloe
Personal Care Hand Sanitizer Antibacterial Aloe
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredient Purpose
Ethyl Alcohol 62 percent Antimicrobial
Purpose
Uses
hand sanitizer to help reduce bacteria on the skin.
Warnings
For external use only.
Flammable
Keep away from heat and flame.
When using this product avoid contact with face, eyes,
and broken skin. In case of eye contact, flush with plenty
of water and seek medical advice.
Stop use and ask a doctor if irritation or redness develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center.
Directions
wet hands thoroughly with product and rub into skin until dry.
Children under 6 years of age should be supervised by an adult when using this product.
Inactive ingredients
aloe barbadensis leaf juice,
carbomer, fragrance, glycerin, propylene glycol , tocopheryl acetate vitamin E,
triethanolamine, water aqua, FD and C blue 1, FD and C Yellow 5
Kills 99.9 percent of many common germs.
Distributed by Personal Care Products, Inc.
Bingham Farms, MI 48025 3463
Made in China
Personal Care
Kills 99.9 percent Of Germs
Hand Sanitizer
Antibacterial
with Moisturizers
and Aloe
12 Fl oz 355 ml
Personal Care Hand Sanitizer AloeALCOHOL GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||