Perphenazine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- PERPHENAZINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PERPHENAZINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- INFORMATION FOR PATIENTS
- PREGNANCY
- GERIATRIC USE
- PERPHENAZINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGIncreased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine is not approved for the treatment of patients with dementia-related psychosis (seeWARNINGS).
PERPHENAZINE DESCRIPTION
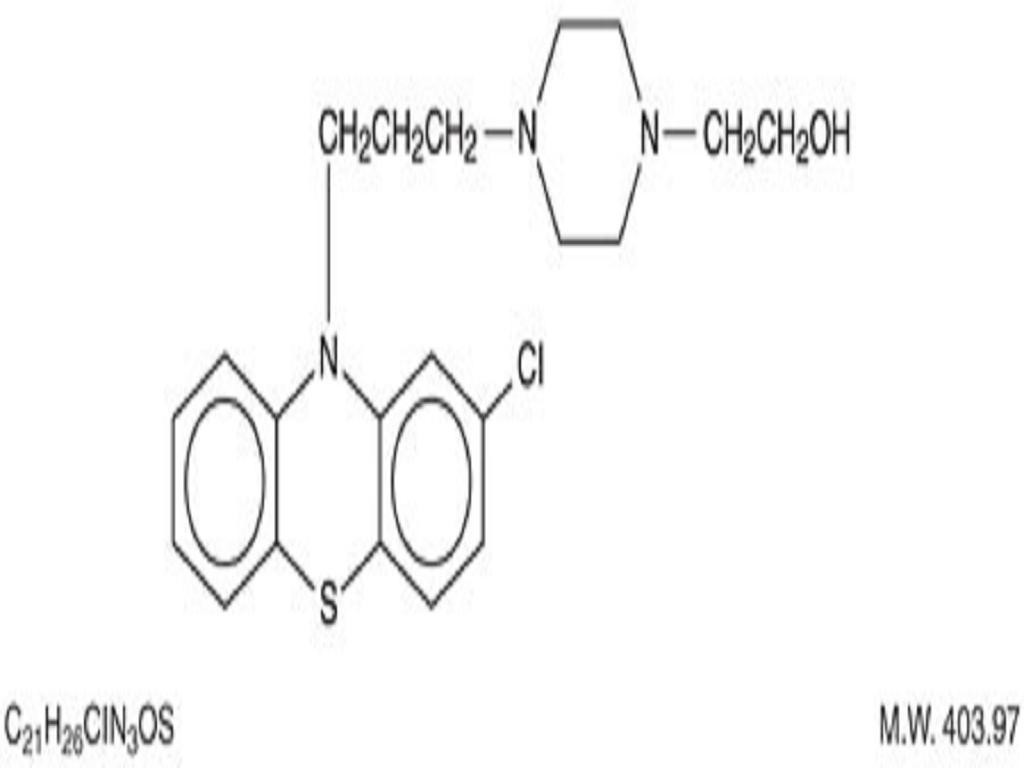
ACTIONS
CLINICAL PHARMACOLOGY
PharmacokineticsINDICATIONS & USAGE
PERPHENAZINE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisElderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Perphenazine is not approved for the treatment of patients with dementia-related psychosis (seeBOXED WARNING).
Information for PatientsADVERSE REACTIONS
Neuroleptic Malignant Syndrome (NMS)
Usage in Pregnancy
PRECAUTIONS
DRUG INTERACTIONS
INFORMATION FOR PATIENTS
Leukopenia, Neutropenia and Agranulocytosis
PREGNANCY
GERIATRIC USE
WARNINGS
PERPHENAZINE ADVERSE REACTIONS
CNS Effects
Dystonia
Persistent Tardive Dyskinesia
Other CNS Effects
WARNINGS
Autonomic Effects
Allergic Effects
Endocrine Effects
Cardiovascular Effects
Hematological Effects
Other Effects
OVERDOSAGE
Manifestations
ADVERSE REACTIONS
Treatment
Treatment is symptomatic and supportive. Induction of emesis is not recommended because of the possibility of a seizure, CNS depression, or dystonic reaction of the head or neck and subsequent aspiration. Gastric lavage (after intubation, if the patient is unconscious) and administration of activated charcoal together with a laxative should be considered. There is no specific antidote.
Standard measures (oxygen, intravenous fluids, corticosteroids) should be used to manage circulatory shock or metabolic acidosis. An open airway and adequate fluid intake should be maintained. Body temperature should be regulated. Hypothermia is expected, but severe hyperthermia may occur and must be treated vigorously (seeCONTRAINDICATIONS).
An electrocardiogram should be taken and close monitoring of cardiac function instituted if there is any sign of abnormality. Close monitoring of cardiac function is advisable for not less than five days. Vasopressors such as norepinephrine may be used to treat hypotension, but epinephrine should NOT be used.
Hemodialysis and peritoneal dialysis are of no value because of low plasma concentrations of the drug.
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase.
DOSAGE & ADMINISTRATION
Moderately disturbed nonhospitalized patients with schizophrenia
Hospitalized patients with schizophrenia
Severe nausea and vomiting in adults
Elderly Patients
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PerphenazinePerphenazine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!