Pentazocine and naloxone
Lake Erie Medical & Surgical Supply DBA Quality Care Products LLC
Revised: June 2008 173017 CIV
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
| Pentazocine and naloxone tablets are intended for oral use only. Severe, potentially lethal, reactions may result from misuse of pentazocine and naloxone tablets by injection either alone or in combination with other substances. (See DRUG ABUSE AND DEPENDENCE section.) |
Pentazocine and naloxone tablets USP contain pentazocine hydrochloride USP, equivalent to 50 mg base, a member of the benzazocine series (also known as the benzomorphan series), and naloxone hydrochloride USP, equivalent to 0.5 mg base.
Pentazocine and naloxone tablets are an analgesic for oral administration.
Chemically, pentazocine hydrochloride is (2R*, 6R*, 11R*)-1,2,3,4,5,6-Hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol hydrochloride, a white, crystalline substance soluble in acidic aqueous solutions. It has the following structural formula:
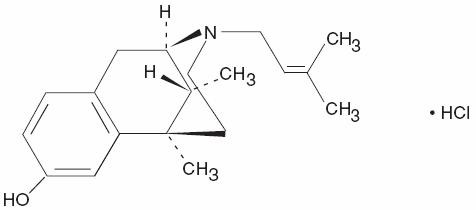
C19H21N04•HCI Molecular Weight 363.84
Each tablet, for oral administration, contains pentazocine hydrochloride USP, equivalent to 50 mg of pentazocine, and naloxone hydrochloride USP, equivalent to 0.5 mg of naloxone. In addition each tablet contains the following inactive ingredients: colloidal silicon dioxide, dibasic calcium phosphate, D&C Yellow No. 10 Al-lake, FD&C Blue No. 1 Al-lake, FD&C Yellow No. 6 Al-lake, magnesium stearate, microcrystalline cellulose, pregelatinized starch, and sodium lauryl sulfate.
Pentazocine is a potent analgesic which when administered orally in a 50 mg dose appears equivalent in analgesic effect to 60 mg (1 grain) of codeine. Onset of significant analgesia usually occurs between 15 and 30 minutes after oral administration, and duration of action is usually three hours or longer. Onset and duration of action and the degree of pain relief are related both to dose and the severity of pretreatment pain. Pentazocine weakly antagonizes the analgesic effects of morphine and meperidine; in addition, it produces incomplete reversal of cardiovascular, respiratory, and behavioral depression induced by morphine and meperidine. Pentazocine has about 1/50 the antagonistic activity of nalorphine. It also has sedative activity.
Pentazocine is well absorbed from the gastrointestinal tract. Concentrations in plasma coincide closely with the onset, duration, and intensity of analgesia; peak values occur 1 to 3 hours after oral administration. The half-life in plasma is 2 to 3 hours.
Pentazocine is metabolized in the liver and excreted primarily in the urine. Pentazocine passes into the fetal circulation.
Naloxone when administered orally at 0.5 mg has no pharmacologic activity. Naloxone hydrochloride administered parenterally at the same dose is an effective antagonist to pentazocine and a pure antagonist to narcotic analgesics.
Pentazocine and naloxone tablets are a potent analgesic when administered orally. However the presence of naloxone in pentazocine and naloxone tablets will prevent the effect of pentazocine if the product is misused by injection.
Studies in animals indicate that the presence of naloxone does not affect pentazocine analgesia when the combination is given orally. If the combination is given by injection the action of pentazocine is neutralized.
| Pentazocine and naloxone tablets are intended for oral use only. Severe, potentially lethal, reactions may result from misuse of pentazocine and naloxone tablets by injection either alone or in combination with other substances. (See DRUG ABUSE AND DEPENDENCE section.) |
Pentazocine and naloxone tablets are indicated for the relief of moderate to severe pain.
Pentazocine and naloxone tablets are indicated for oral use only.
Pentazocine and naloxone tablets should not be administered to patients who are hypersensitive to either pentazocine or naloxone.
| Pentazocine and naloxone tablets are intended for oral use only. Severe, potentially lethal, reactions may result from misuse of pentazocine and naloxone tablets by injection either alone or in combination with other substances. (See DRUG ABUSE AND DEPENDENCE section.) |
Drug Dependence. Pentazocine can cause a physical and psychological dependence. (See DRUG ABUSE AND DEPENDENCE.)
Head Injury and Increased Intracranial Pressure. As in the case of other potent analgesics, the potential of pentazocine for elevating cerebrospinal fluid pressure may be attributed to CO2 retention due to the respiratory depressant effects of the drug. These effects may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increase in intracranial pressure. Furthermore, pentazocine can produce effects which may obscure the clinical course of patients with head injuries. In such patients, pentazocine must be used with extreme caution and only if its use is deemed essential.
Usage with Alcohol. Due to the potential for increased CNS depressant effects, alcohol should be used with caution in patients who are currently receiving pentazocine.
Patients Receiving Narcotics. Pentazocine is a mild narcotic antagonist. Some patients previously given narcotics, including methadone for the daily treatment of narcotic dependence, have experienced withdrawal symptoms after receiving pentazocine.
Certain Respiratory Conditions. Although respiratory depression has rarely been reported after oral administration of pentazocine, the drug should be administered with caution to patients with respiratory depression from any cause, severely limited respiratory reserve, severe bronchial asthma, and other obstructive respiratory conditions, or cyanosis.
Acute CNS Manifestations. Patients receiving therapeutic doses of pentazocine have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be very closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur.
Caution should be used when pentazocine is administered to patients prone to seizures; seizures have occurred in a few such patients in association with the use of pentazocine though no cause and effect relationship has been established.
Decreased metabolism of pentazocine by the liver in extensive liver disease may predispose to accentuation of side effects. Although laboratory tests have not indicated that pentazocine causes or increases renal or hepatic impairment, the drug should be administered with caution to patients with such impairment.
In prescribing pentazocine for long-term use, the physician should take precautions to avoid increases in doses by the patient.
Narcotic drug products are generally considered to elevate biliary tract pressure for varying periods following their administration. Some evidence suggests that pentazocine may differ from other marketed narcotics in this respect (i.e., it causes little or no elevation in biliary tract pressures). The clinical significance of these findings, however, is not yet known.
Since sedation, dizziness, and occasional euphoria have been noted, ambulatory patients should be warned not to operate machinery, drive cars, or unnecessarily expose themselves to hazards. Pentazocine may cause physical and psychological dependence when taken alone and may have additive CNS depressant properties when taken in combination with alcohol or other CNS depressants.
As with all drugs, pentazocine should be used with caution in patients with myocardial infarction who have nausea or vomiting.
Usage with Alcohol: See WARNINGS.
No long-term studies in animals to test for carcinogenesis have been performed with the components of pentazocine and naloxone hydrochlorides tablets.
Animal reproduction studies have not been conducted with pentazocine and naloxone tablets. It is also not known whether pentazocine and naloxone tablets can cause fetal harm when administered to pregnant women or can affect reproduction capacity. Pentazocine and naloxone tablets should be given to pregnant women only if clearly needed. However, animal reproduction studies with pentazocine have not demonstrated teratogenic or embryotoxic effects.
Patients receiving pentazocine during labor have experienced no adverse effects other than those that occur with commonly used analgesics. Pentazocine and naloxone tablets should be used with caution in women delivering premature infants. The effect of pentazocine and naloxone tablets on the mother and fetus, the duration of labor and delivery, the possibility that forceps delivery or other intervention or resuscitation of the newborn may be necessary, or the effect of pentazocine and naloxone tablets on the later growth, development, and functional maturation of the child are unknown at the present time.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when pentazocine and naloxone tablets are administered to a nursing woman.
Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Controlled clinical studies of pentazocine and naloxone tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses or effectiveness in analgesic activity between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Cardiovascular: Hypotension, tachycardia, syncope.
Respiratory: Rarely, respiratory depression.
Acute CNS Manifestations: Patients receiving therapeutic doses of pentazocine have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted it should be done with caution since these acute CNS manifestations may recur.
Other CNS Effects: Dizziness, lightheadedness, hallucinations, sedation, euphoria, headache, confusion, disorientation; infrequently weakness, disturbed dreams, insomnia, syncope, visual blurring and focusing difficulty, depression; and rarely tremor, irritability, excitement, tinnitus.
Autonomic: Sweating; infrequently flushing; and rarely chills.
Gastrointestinal: Nausea, vomiting, constipation, diarrhea, anorexia, rarely abdominal distress.
Allergic: Edema of the face; dermatitis, including pruritus; flushed skin, including plethora; infrequently rash, and rarely urticaria.
Ophthalmic: Visual blurring and focusing difficulty.
Hematologic: Depression of white blood cells (especially granulocytes), which is usually reversible, moderate transient eosinophilia.
Other: Headache, chills, insomnia, weakness, urinary retention, paresthesia, serious skin reactions, including erythema multiforme, Stevens-Johnson Syndrome and toxic epidermal necrolysis.
Controlled Substance. Pentazocine and naloxone tablets are a Schedule IV controlled substance.
There have been some reports of dependence and of withdrawal symptoms with orally administered pentazocine. Patients with a history of drug dependence should be under close supervision while receiving pentazocine orally. There have been rare reports of possible abstinence syndromes in newborns after prolonged use of pentazocine during pregnancy.
There have been instances of psychological and physical dependence on parenteral pentazocine in patients with a history of drug abuse and rarely, in patients without such a history. Abrupt discontinuance following the extended use of parenteral pentazocine has resulted in withdrawal symptoms.
In prescribing pentazocine for chronic use, the physician should take precautions to avoid increases in dose by the patient.
The amount of naloxone present in pentazocine and naloxone tablets (0.5 mg per tablet) has no action when taken orally and will not interfere with the pharmacologic action of pentazocine. However, this amount of naloxone given by injection has profound antagonistic action to narcotic analgesics.
Severe, even lethal, consequences may result from misuse of tablets by injection either alone or in combination with other substances, such as pulmonary emboli, vascular occlusion, ulceration and abscesses, and withdrawal symptoms in narcotic dependent individuals.
Pentazocine and naloxone tablets contain an opioid antagonist, naloxone (0.5 mg). Naloxone is inactive when administered orally at this dose, and its inclusion in pentazocine and naloxone tablets is intended to curb a form of misuse of oral pentazocine. Parenterally, naloxone is an active narcotic antagonist. Thus, pentazocine and naloxone tablets have a lower potential for parenteral misuse than the previous oral pentazocine hydrochloride formulation. However, it is still subject to patient misuse and abuse by the oral route.
Manifestations. Clinical experience of overdosage with this oral medication has been insufficient to define the signs of this condition.
Treatment. Oxygen, intravenous fluids, vasopressors, and other supportive measures should be employed as indicated. Assisted or controlled ventilation should also be considered. For respiratory depression due to overdosage or unusual sensitivity to pentazocine, parenteral naloxone is a specific and effective antagonist.
| Pentazocine and naloxone tablets are intended for oral use only. Severe, potentially lethal, reactions may result from misuse of pentazocine and naloxone tablets by injection either alone or in combination with other substances. (See DRUG ABUSE AND DEPENDENCE section.) |
Adults. The usual initial adult dose is 1 tablet every three or four hours. This may be increased to 2 tablets when needed. Total daily dosage should not exceed 12 tablets.
When anti-inflammatory or antipyretic effects are desired in addition to analgesia, aspirin can be administered concomitantly with this product.
Pediatric Patients. Since clinical experience in pediatric patients under 12 years of age is limited, administration of this product in this age group is not recommended.
Duration of Therapy. Patients with chronic pain who receive pentazocine and naloxone tablets orally for prolonged periods have only rarely been reported to experience withdrawal symptoms when administration was abruptly discontinued (see WARNINGS). Tolerance to the analgesic effect of pentazocine has also been reported only rarely. However, there is no long-term experience with the oral administration of pentazocine and naloxone tablets.
Pentazocine and Naloxone Tablets USP are light green, scored, capsule shaped tablets debossed 395 to the left of the score, 50 over 0.5 to the right of the score and WATSON on the reverse side supplied in bottles of 100.
Store at 20°-25°C (68°-77°F). [See USP controlled room temperature.]
Dispense in a tight, light-resistant container as defined in the USP.
Manufactured by: Watson Laboratories, Inc.
Corona, CA 92880 USA
Distributed by: Watson Pharma, Inc.
Corona, CA 92880 USA
Revised: June 2008 0608B
173017
Image of Label

Pentazocine and naloxonePentazocine hydrochloride and naloxone hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||