Panretin
(For topical use only)
FULL PRESCRIBING INFORMATION: CONTENTS*
- PANRETIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- PANRETIN INDICATIONS AND USAGE
- PANRETIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PANRETIN ADVERSE REACTIONS
- OVERDOSAGE
- PANRETIN DOSAGE AND ADMINISTRATION
- How Supplied
FULL PRESCRIBING INFORMATION
DESCRIPTION
Panretin® gel 0.1% contains alitretinoin and is intended for topical application only. The chemical name is 9-cis-retinoic acid and the structural formula is as follows:
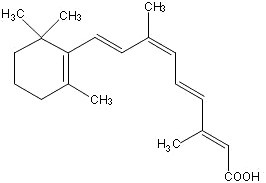
Chemically, alitretinoin is related to vitamin A. It is a yellow powder with a molecular weight of 300.44 and a molecular formula of C20H28O2. It is slightly soluble in ethanol (7.01 mg/g at 25°C) and insoluble in water. Panretin® gel is a clear, yellow gel containing 0.1% (w/w) alitretinoin in a base of dehydrated alcohol USP, polyethylene glycol 400 NF, hydroxypropyl cellulose NF, and butylated hydroxytoluene NF.
CLINICAL PHARMACOLOGY
Mechanism of Action
Alitretinoin (9-cis-retinoic acid) is a naturally-occurring endogenous retinoid that binds to and activates all known intracellular retinoid receptor subtypes (RARα, RARβ, RARγ, RXRα, RXRβ and RXRγ). Once activated these receptors function as transcription factors that regulate the expression of genes that control the process of cellular differentiation and proliferation in both normal and neoplastic cells. Alitretinoin inhibits the growth of Kaposi's sarcoma (KS) cells in vitro.
Pharmacokinetics
No studies have examined plasma 9-cis-retinoic acid concentrations before and after treatment with Panretin® gel. There is, however, indirect evidence that absorption is not extensive. Plasma concentrations of 9-cis-retinoic acid were evaluated during clinical studies in patients with cutaneous lesions of AIDS-related KS after repeated multiple-daily dose application of Panretin® gel for up to 60 weeks. The range of 9-cis-retinoic acid plasma concentrations in these patients was similar to the range of circulating, naturally-occurring 9-cis-retinoic acid plasma concentrations in untreated healthy volunteers.
Although there are no detectable plasma concentrations of 9-cis-retinoic acid metabolites after topical application of Panretin® gel, in vitro studies indicate that the drug is metabolized to 4-hydroxy-9-cis-retinoic acid and 4-oxo-9-cis-retinoic acid by CYP 2C9, 3A4, 1A1, and 1A2 enzymes. In vivo, 4-oxo-9-cis-retinoic acid is the major circulating metabolite following oral administration of 9-cis-retinoic acid.
No formal pharmacokinetic drug interaction studies between Panretin® gel and antiretroviral agents have been conducted.
Clinical Studies
Panretin® gel is not a systemic therapy; it therefore cannot treat visceral Kaposi's sarcoma (KS) nor prevent the development of new KS lesions where it has not been applied. Visceral KS disease was not monitored in these trials, and the appearance of new KS lesions was not considered part of the response assessment in clinical trials.
Panretin® gel was evaluated in two multicenter, prospective, randomized, double-blind, vehicle-controlled studies in patients with cutaneous lesions of AIDS-related KS. In both studies the primary efficacy endpoint was the patients' cutaneous KS tumor response rate through 12 weeks of study drug treatment which was assessed by evaluating from 3 to 8 KS index lesions according to the modified AIDS Clinical Trials Group (ACTG) response criteria as applied to topical therapy (i.e., evaluation of height and area reductions of the index lesions only; progressive disease in non-index lesions and new lesions were not considered progressive disease; progressive disease was scored only in the treated index lesions). A global evaluation by physicians was also carried out. It considered all of the patient's treated lesions (index and other) compared to baseline. In this evaluation, patients with at least a 50% improvement in the KS lesions were considered responders. In addition, photographs of lesions in patients considered responders by the modified ACTG criteria were examined by the FDA for a cosmetically beneficial response, defined as at least a 50% improvement in appearance compared to baseline, considering both the KS lesions and dermal toxicity at the lesion site, in at least 50% of the index lesions and maintained for at least 3 weeks. Patients were also asked about their satisfaction with the treatment.
In Study 1, a total of 268 patients were entered from centers in the U.S. and Canada. Patients were treated topically three to four times a day with either Panretin® gel or a matching vehicle gel for a minimum of 12 weeks, followed by an open-label phase in patients who had not yet progressed on Panretin® gel. Responses during the double-blind phase are shown in Table 1. Responses to Panretin® gel were seen in both previously untreated patients and in patients with prior systemic and/or topical KS treatment. A total of 72 patients responded to Panretin( gel during the randomized or crossover portions of the study. At a median duration of monitoring of 16 weeks, only 15% of the 72 patients had relapsed. Panretin( gel would not be expected to affect development of new lesions in untreated areas and these were seen in about 50% of patients, at similar rates in treated and untreated patients, responders and non-responders. The patients' assessment of their overall satisfaction with the drug effect on all treated lesions significantly favored Panretin® gel.
Study 2 was an international study with a planned enrollment of 270 patients. Patients were treated topically twice a day with Panretin® gel or a matching vehicle for 12 weeks. The study was stopped early because of positive interim results in the initial 82 patient data set. Results of the study are shown in Table 1. Responses to Panretin® gel were seen both in previously untreated patients and in patients with prior systemic and/or topical KS treatment.
| STUDY 1 | STUDY 2 | |||
|
Panretin® Gel N=134 |
Vehicle Gel N=134 |
Panretin® Gel N=36 |
Vehicle Gel N=46 |
|
|
Modified ACTG Response (index lesions) |
34% PR 1% CR |
16% PR p=0.0012 |
36% PR | 7% PR |
|
Physician's Global/Subjective Assessment (all treated lesions) |
19% PR |
4% PR p=0.00014 |
47% PR | 11% PR |
|
Beneficial Response Photographs (index lesions only) |
15% |
4% p=0.0026 |
19% | 2% |
In the clinical trials, responses were seen as early as two (2) weeks; most patients, however, required four (4) to eight (8) weeks of treatment, and some patients did not experience significant improvement until 14 or more weeks of treatment. The cumulative percentage of patients who achieved a response was less than 1% at 2 weeks, 10% at 4 weeks, and 28% at 8 weeks.
In both studies, responses occurred in patients with a wide range of baseline CD4+ lymphocyte counts, including patients with CD4+ lymphocyte counts less than 50 cells/mm3. Nearly all patients received concomitant combination antiretroviral therapy.
Photographs of patients revealed a substantial erythematous and edematous response in some cases, leading to a cosmetically mixed outcome even in apparent responders. Nonetheless, in Study 1 it appeared that a cosmetically satisfactory result occurred at about the same rate as the Physician's Global response rate and in both studies such a response was more frequent than in the vehicle control.
INDICATIONS AND USAGE
Panretin® gel is indicated for topical treatment of cutaneous lesions in patients with AIDS-related Kaposi's sarcoma. Panretin® gel is not indicated when systemic anti-KS therapy is required (e.g., more than 10 new KS lesions in the prior month, symptomatic lymphedema, symptomatic pulmonary KS, or symptomatic visceral involvement). There is no experience to date using Panretin® gel with systemic anti-KS treatment.
CONTRAINDICATIONS
Panretin® gel is contraindicated in patients with a known hypersensitivity to retinoids or to any of the ingredients of the product.
WARNINGS
Pregnancy
Panretin® gel could cause fetal harm if significant absorption were to occur in a pregnant woman. 9-cis-Retinoic acid has been shown to be teratogenic in rabbits and mice. An increased incidence of fused sternebrae and limb and craniofacial defects occurred in rabbits given oral doses of 0.5 mg/kg/day (about five times the estimated daily human topical dose on a mg/m2 basis, assuming complete systemic absorption of 9-cis-retinoic acid, when Panretin® gel is administered as a 60 g tube over 1 month in a 60 kg human) during the period of organogenesis. Limb and craniofacial defects also occurred in mice given a single oral dose of 50 mg/kg on day eleven of gestation (about 127 times the estimated daily human topical dose on a mg/m2 basis). Oral 9-cis-retinoic acid was also embryocidal, as indicated by early resorptions and post-implantation loss when it was given during the period of organogenesis to rabbits at doses of 1.5 mg/kg/day (about 15 times the estimated daily human topical dose on a mg/m2 basis) and to rats at doses of 5 mg/kg/day (about 25 times the estimated daily human topical dose on a mg/m2 basis). Animal reproduction studies with topical 9-cis-retinoic acid have not been conducted. It is not known whether topical Panretin® gel can modulate endogenous 9-cis-retinoic acid levels in a pregnant woman nor whether systemic exposure is increased by application to ulcerated lesions or by duration of treatment. There are no adequate and well-controlled studies in pregnant women. If Panretin® gel is used during pregnancy, or if the patient becomes pregnant while taking it, the patient should be apprised of the potential hazard to the fetus. Women of child-bearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
Panretin® gel is indicated for topical treatment of Kaposi's sarcoma. Patients with cutaneous T-cell lymphoma were less tolerant of topical Panretin® gel; five of seven patients had 6 episodes of treatment-limiting toxicities—grade 3 dermal irritation—with Panretin® gel (0.01% or 0.05%).
Information for Patients
Please see accompanying "Patient's Instructions for Use"
Photosensitivity
Retinoids as a class have been associated with photosensitivity. There were no reports of photosensitivity associated with the use of Panretin® gel in the clinical studies. Nonetheless, because in vitro data indicate that 9-cis-retinoic acid may have a weak photosensitizing effect, patients should be advised to minimize exposure of treated areas to sunlight and sunlamps during the use of Panretin® gel.
Drug Interactions
Patients who are applying Panretin® gel should not concurrently use products that contain DEET (N,N-diethyl-m-toluamide), a common component of insect repellent products. Animal toxicology studies showed increased DEET toxicity when DEET was included as part of the formulation.
Although there was no clinical evidence in the vehicle-controlled studies of drug interactions with systemic antiretroviral agents, including protease inhibitors, macrolide antibiotics, and azole antifungals, the effect of Panretin® gel on the steady-state concentrations of these drugs is not known. No drug interaction data are available on concomitant administration of Panretin® gel and systemic anti-KS agents.
Drug/Laboratory Test Interactions
No interference with laboratory tests has been observed.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to assess the carcinogenic potential of 9-cis-retinoic acid have not been conducted. 9-cis-Retinoic acid was not mutagenic in vitro (bacterial assays, Chinese hamster ovary cell HGPRT mutation assay) and was not clastogenic in vitro (chromosome aberration test in human lymphocytes) nor in vivo (mouse micronucleus test).
Pregnancy Category D
(see "Warnings" section)
Nursing Mothers
It is not known whether alitretinoin or its metabolites are excreted in human milk. Because many drugs are excreted in human milk and because of the potential for adverse reactions from Panretin® gel in nursing infants, mothers should discontinue nursing prior to using the drug.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Inadequate information is available to assess safety and efficacy in patients age 65 years or older.
ADVERSE REACTIONS
The safety of Panretin® gel has been assessed in clinical studies of 385 patients with AIDS-related KS. Adverse events associated with the use of Panretin® gel in patients with AIDS-related KS occurred almost exclusively at the site of application. The dermal toxicity begins as erythema; with continued application of Panretin® gel, erythema may increase and edema may develop. Dermal toxicity may become treatment-limiting, with intense erythema, edema, and vesiculation. Usually, however, adverse events are mild to moderate in severity; they led to withdrawal from the study in only 7% of the patients. Severe local (application site) skin adverse events occurred in about 10% of patients in the U.S. study (versus 0% in the vehicle control). Table 2 lists the adverse events that occurred at the application site with an incidence of at least 5% during the double-blind phase in the Panretin® gel-treated group and in the vehicle control group in either of the two controlled studies. Adverse events were reported at other sites but generally were similar in the two groups.
|
Includes Investigator terms: 1 Erythema, scaling, irritation, redness, rash, dermatitis 2 Burning, pain 3 Itching, pruritus 4 Flaking, peeling, desquamation, exfoliation 5 Excoriation, cracking, scab, crusting, drainage, eschar, fissure or oozing 6 Stinging, tingling 7 Edema, swelling, inflammation |
||||
| Adverse Event Term | Study 1 | Study 2 | ||
|
Panretin® Gel N=134 Pts. % |
Vehicle Gel N=134 Pts. % |
Panretin® Gel N=36 Pts. % |
Vehicle Gel N=46 Pts. % |
|
| Rash1 | 77 | 11 | 25 | 4 |
| Pain2 | 34 | 7 | 0 | 4 |
| Pruritus3 | 11 | 4 | 8 | 4 |
| Exfoliative dermatitis4 | 9 | 2 | 3 | 0 |
| Skin disorder5 | 8 | 1 | 0 | 0 |
| Paresthesia6 | 3 | 0 | 22 | 7 |
| Edema7 | 8 | 3 | 3 | 0 |
OVERDOSAGE
There has been no experience with acute overdose of Panretin® gel in humans. Systemic toxicity following acute overdosage with topical application of Panretin® gel is unlikely because of limited systemic plasma levels observed with normal therapeutic doses. There is no specific antidote for overdosage.
DOSAGE AND ADMINISTRATION
Panretin® gel should initially be applied two (2) times a day to cutaneous KS lesions. The application frequency can be gradually increased to three (3) or four (4) times a day according to individual lesion tolerance. If application site toxicity occurs, the application frequency can be reduced. Should severe irritation occur, application of drug can be temporarily discontinued for a few days until the symptoms subside.
Sufficient gel should be applied to cover the lesion with a generous coating. The gel should be allowed to dry for three to five minutes before covering with clothing. Because unaffected skin may become irritated, application of the gel to normal skin surrounding the lesions should be avoided. In addition, do not apply the gel on or near mucosal surfaces of the body.
A response of KS lesions may be seen as soon as two weeks after initiation of therapy but most patients require longer application. With continued application, further benefit may be attained. Some patients have required over 14 weeks to respond. In clinical trials, Panretin® gel was applied for up to 96 weeks. Panretin® gel should be continued as long as the patient is deriving benefit.
Occlusive dressings should not be used with Panretin® gel.
How Supplied
Panretin® gel is available in tubes containing 60 grams, (60 mg active ingredient alitretinion). NDC 62856-601-22
Store at 25° C (77° F); excursions permitted to 15-30° C (59-86° F) [see USP Controlled Room Temperature].
Manufactured for:
Eisai Inc.
Woodcliff Lake, NJ 07677
by:
Manufactured by:
DPT Laboratories, Ltd.
318 McCullough
San Antonio, TX 78215
Panretin® is a registered trademark of Eisai Inc.
© 2012 Eisai Inc.
Panretin®
(alitretinoin)
gel 0.1%
Patient's Instructions for Use
(For topical use only)
Your health care provider has prescribed Panretin® gel for the management of the Kaposi's sarcoma (KS) lesions on your skin. The following simple instructions will help you successfully begin and continue your treatment.
WARNINGS
DO NOT apply gel on or near mucosal surfaces of the body such as eyes, nostrils, mouth, lips, vagina, tip of the penis, rectum or anus.
DO NOT use insect repellents containing DEET (N,N-diethyl-m-toluamide) or other products containing DEET while using Panretin® gel.
Keep Out of Reach of Children.
Product contains alcohol and should be kept away from open flame.
DO NOT use Panretin® gel if you are pregnant or breastfeeding. Precautions should be taken to avoid becoming pregnant while using Panretin® gel. If you are pregnant, thinking of becoming pregnant, or breastfeeding, speak with your health care provider for more information.
Topical Panretin® gel does not treat lung or intestinal Kaposi's sarcoma.
Topical Panretin® gel does not prevent the appearance of new KS lesions or the
increased growth of KS lesions not treated with Panretin® gel.
Topical Panretin® gel does not treat extremity swelling associated with KS. It is important to understand that KS lesions can appear and affect other parts of your body, including internal organs (e.g., lungs and intestines). You should regularly consult your health care provider about the status of your KS disease, especially if you note changes.
HOW TO APPLY
Apply Panretin® gel to your KS lesions using a clean finger. Place a generous coating of gel over the entire surface of each lesion that you want to treat. It is not necessary to physically rub the gel into the lesion. You should make every effort not to apply gel to the healthy skin around the lesion. The extra effort you take in carefully applying the gel only to the area of the KS lesion will help to lessen any irritation or redness which may occur. Proper application should leave some gel visible on the surface of the lesion when you are finished with the application.
Immediately following application, wipe the finger(s) you have used to apply the gel with a disposable tissue and wash your hands using soap and water.
Allow the gel to dry before covering a treated area with clothing. This will usually take from three (3) to five (5) minutes.
A mild soap is recommended when bathing or showering.
WHEN TO APPLY
Panretin® gel should be applied at an initial frequency of two (2) times daily. Your health care provider may instruct you to apply Panretin® gel at a different frequency (up to four [4] times daily). Applications should be spaced as evenly as possible throughout the day. If you apply Panretin® gel after your shower or bath, you should wait 20 minutes before application.
YOU SHOULD AVOID...
You should avoid applying the gel to areas of healthy skin around a KS lesion. Exposure of healthy skin to Panretin® gel may cause unnecessary irritation or redness.
You should avoid showering, bathing, or swimming until at least three (3) hours after any application, if possible.
You should avoid covering the KS lesions treated with gel with any bandage or material other than loose clothing.
You should avoid prolonged exposure of the treated area to sunlight or other ultraviolet (UV) light (such as tanning lamps).
You should avoid the use of other topical products on your treated KS lesions. Mineral oil may be used between Panretin® gel applications in order to help prevent excessive dryness or itching. However, mineral oil should not be applied for at least two (2) hours before or after the application of Panretin® gel.
You should avoid scratching the treated areas.
WHAT TO EXPECT
Do not be discouraged if you do not see immediate improvement.
Do not stop treatment at the first sign of improvement.
While using Panretin® gel, you may experience some local effects such as redness, discomfort, itching, and skin peeling or flaking at the area of application. Other possible local skin effects include: rawness, surface or deep cracking, scabbing, crusting, drainage, oozing, or infection. Should these or other effects become troublesome to you, consult your health care provider. He or she can provide information on how to manage these effects.
HOW QUICKLY CAN I EXPECT PANRETIN® GEL TO WORK?
Be patient. Panretin® gel takes time to work, up to 14 weeks or more of treatment. In clinical trials, few patients experienced the onset of response as early as two (2) weeks; most patients who responded required at least four (4) to eight (8) weeks of treatment, and some patients did not experience significant improvement until 14 or more weeks of treatment. Continue to use Panretin® gel as instructed by your health care provider.
OTHER INFORMATION
The opening of the Panretin® gel tube is covered by a metal safety seal. If this seal has been punctured or is not visible when you open the package, DO NOT USE and promptly return the product to your pharmacy or place of purchase.
To open, use the pointed portion of the cap to puncture the metal safety seal.
Always use the cap to close the tube tightly after each use.
Store at room temperature. Keep away from heat.
IF YOU HAVE QUESTIONS...
If you have any questions about your treatment, talk with your health care provider.
Manufactured for:
Eisai Inc.
Woodcliff Lake, NJ 07677
by:
Manufactured by:
DPT Laboratories, Ltd.
318 McCullough
San Antonio, TX 78215
Eisai Inc.
Woodcliff Lake, NJ 07677
Panretin® is a registered trademark of Eisai Inc.
© 2012 Eisai Inc. (Rev.003/12)
PRINCIPAL DISPLAY PANEL
NDC 62856-601-22
Panretin® gel
(alitretinoin) 0.1%
net wt 60 grams

Panretinalitretinoin GEL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||