pain reliever
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- pain reliever Uses
- Warnings
- Directions
- pain reliever Other information
- Inactive ingredients
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
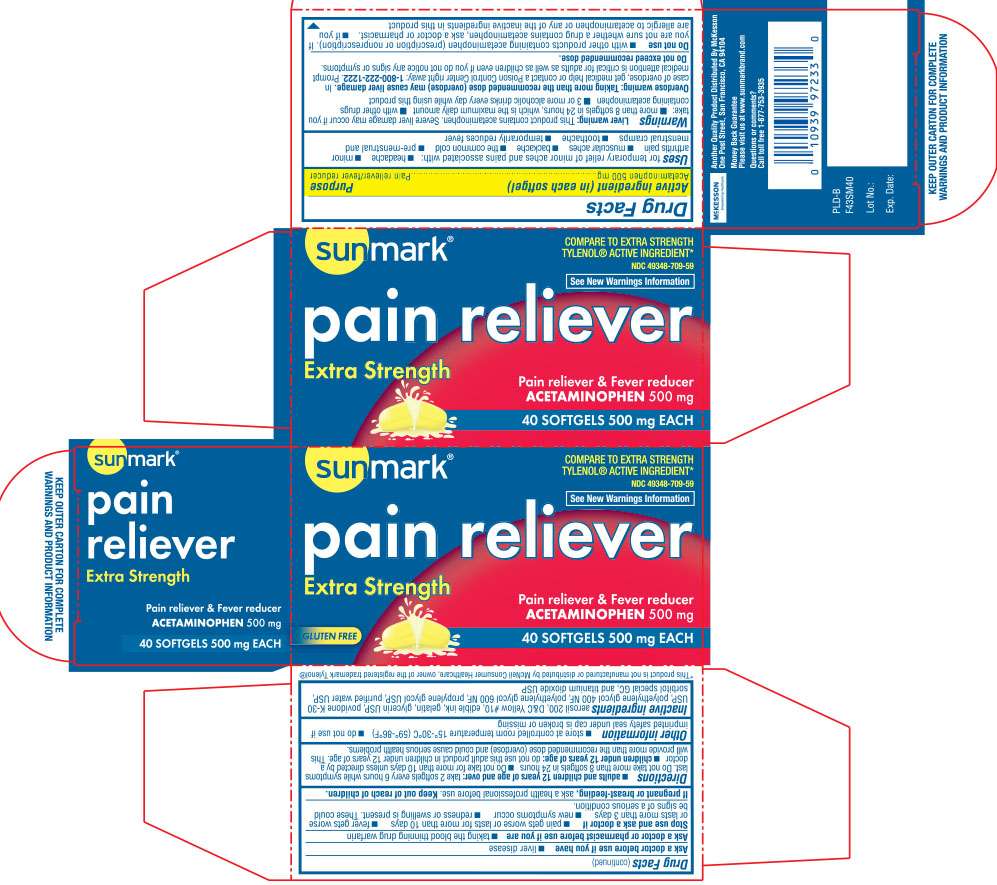
Active ingredient
Acetaminophen 500 mg
Purpose
Pain reliever/ fever reducer
pain reliever Uses
for the temporarily relief of minor aches and pains associated with:
- headache
- minor arthritis pain
- muscular aches
- backache
- the common cold
- pre- menstrual and menstrual cramps
- toothache
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 8 softgels in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks ever day while using this product
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away: 1-800-222-1222. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Do not exceed recommended dose
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor before use if you have
- liver disease
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Directions
- adults and children 12 years and over: take 2 softgels every 6 hours while symptoms last. Do not take more than 8 softgels in 24 hours
- do not take for more than 10 days unless directed by a doctor
- children under 12 years of age: do not use this adult product in children under 12 years of age. This will provide more than the recommended dose (overdose) and could cause serious health problems
pain reliever Other information
- store at controlled room temperature 15°-30°C (59°-86°F)
- do not use if imprinted safety seal under cap is broken or missing
Inactive ingredients
aerosil 200, D&C Yellow #10, edible ink, gelatin, glycerin USP, povidone K- 30 USP, polyethylene glycol 400 NF, polyethylene glycol 600 NF, propylene glycol USP, Purified water USP, sorbitol special GC, and titanium dioxide USP.
Principal Display Panel
Compare to Extra Strength TYLENOL® ACTIVE INGREDIENT*
SEE NEW WARNINGS INFORMATION
PAIN RELIEVER
Extra Strength
pain reliever & fever reducer
ACETAMINOPHEN 500 mg
Questions or comments ?
call toll free 1-877-753-3935
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol®
Product Label
pain relieverAcetaminophen CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||