Pain Relief
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Pain Relief Uses
- Warnings
- Directions
- Pain Relief Other information
- Inactive ingredients
- Carton label
FULL PRESCRIBING INFORMATION
Active ingredient
DL-Camphor 1.2%
L-Menthol 5.7%
Methyl Salicylate 6.3%
Purpose
Topical Analgesic
Topical Analgesic
Topical Analgesic
Pain Relief Uses
- arthritis
- simple backaches
- strains
- bruises
- sprains
Warnings
for external use only
Do not use
- on wounds or damaged skin
- if you are allergic to aspirin or salicylates
- with a heating pad
- with, or at the same time as, other external analgesic products
Ask a doctor before use,
if you are allergic to any ingredients of this product.
When using this product
- use only as directed
- avoid contact with eyes, mucous membranes or rashes
Stop use and ask a doctor if
- rash, itching or excessive skin irritation develops
- conditions worsen
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 12 years of age and over:
- clean and dry affected area
- remove patch from film
- apply to affected area not more than 3 to 4 times daily for 7 days
- wear up to 8 hours per application
- consult a doctor
Pain Relief Other information
- avoid storing product in direct sunlight
- protect from excessive moisture
Inactive ingredients
butylated hydroxytoluene, glyceryl rosinate, natural rubber, polybutene, polyisobutylene, precipitated calcium carbonate, quintone, sorbitan stearate, tocopherol acetate, ys resin, zinc oxide
Carton label
Pain Relief Patch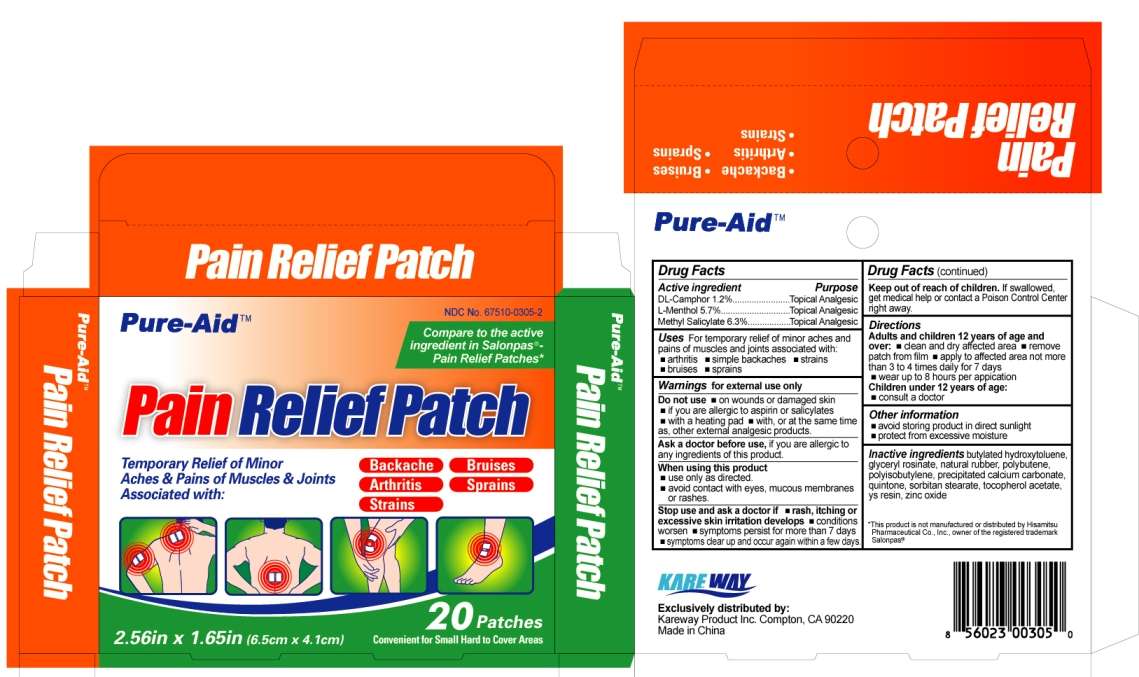
Pain ReliefDL-Camphor, L-Menthol, Methyl Salicylate patch PATCH
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!