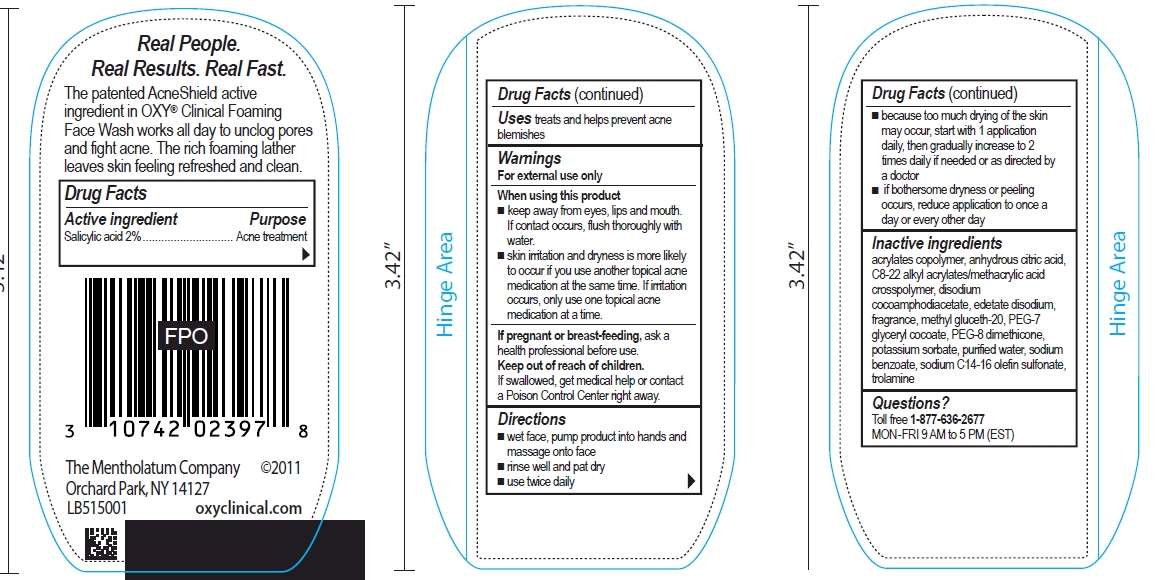
OXY Clinical Foaming Face Wash
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- OXY Clinical Foaming Face Wash Uses
- Warnings
- If pregnant or breast-feeding,
- Keep Out of Reach of Children.
- Directions
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
- Package/Label Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient
Salicylic acid 2%
Purpose
Acne treatment
OXY Clinical Foaming Face Wash Uses
treats and help prevent acne blemishes
Warnings
For external use only
When using this product
- keep away from eyes, lips and mouth. If contact occurs, flush thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
If pregnant or breast-feeding,
ask a health professional before use.
Keep Out of Reach of Children.
If swallowed, contact a Poison Control Center right away.
Directions
- wet face, pump product into hands and massage onto face
- rinse well and pat dry
- use twice daily
- because too much drying of the skin may occur, start with 1 application daily, then gradually increase to 2 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
Inactive ingredients
acrylates copolymer, anhydrous citric acid, C8-22 alkyl acrylates/methycrylic acid crosspolymer, disodium cocoamphodiacetate, edetate disodium, fragrance, methyl gluceth-20, PEG-7 glyceryl cocoate, PEG-8 dimethicone, potassium sorbate, purified water, sodium benzoate, sodium C14-16 olefin sulfonate, trolamine
Questions?
Toll free 1-877-636-2677 MON - FRI 9 AM to 5 PM (EST)
Package/Label Principal Display Panel

OXY Clinical Foaming Face Wash
Acne Treatment 2% Salicylic Acid
Package/Label Principal Display Panel
The Mentholatum Company
Orchard Park NY, 14127
OXY Clinical Foaming Face WashSalicylic Acid SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!