Oxazepam
FULL PRESCRIBING INFORMATION: CONTENTS*
- OXAZEPAM DESCRIPTION
- CLINICAL PHARMACOLOGY
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- INDICATIONS & USAGE
- OXAZEPAM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- PEDIATRIC USE
- GERIATRIC USE
- OXAZEPAM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
OXAZEPAM DESCRIPTION
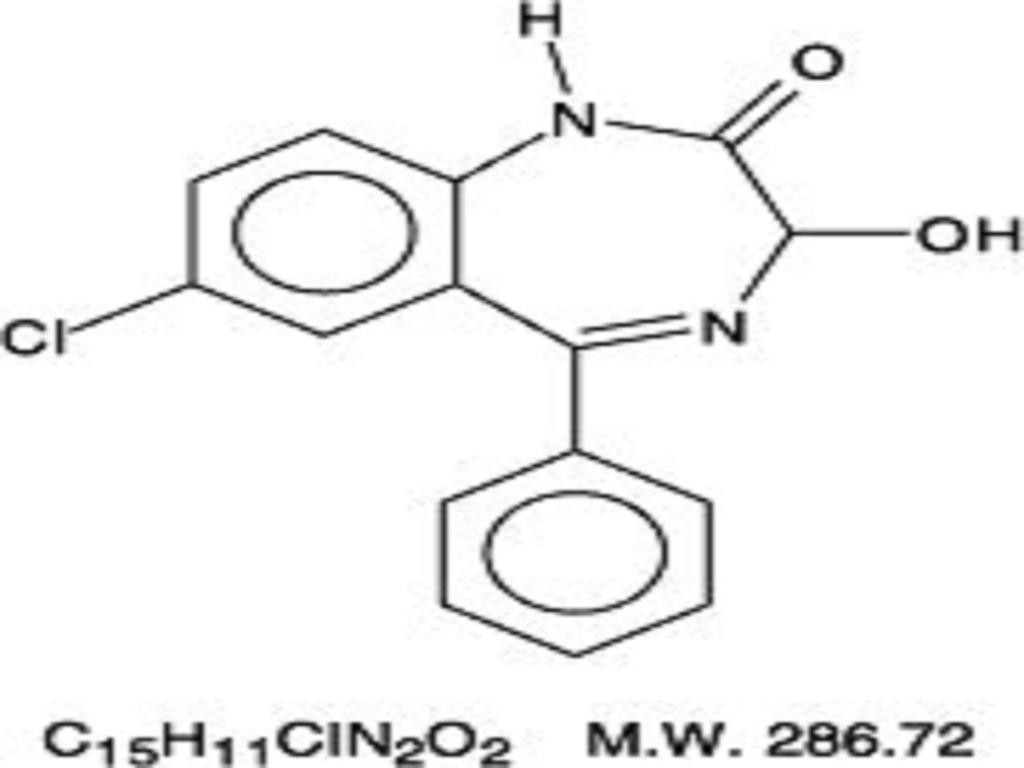
CLINICAL PHARMACOLOGY
PRECAUTIONS: Geriatric Use
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
INDICATIONS & USAGE
OXAZEPAM CONTRAINDICATIONS
WARNINGS
Physical and Psychological Dependence
USE IN PREGNANCY
An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobramate) during the first trimester of pregnancy has been suggested in several studies. Oxazepam, a benzodiazepine derivative, has not been studied adequately to determine whether it, too, may be associated with an increased risk of fetal abnormality. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant, they should communicate with their physician about the desirability of discontinuing the drug.
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGYPRECAUTIONS: GeneralADVERSE REACTIONS). In general, dose selection for oxazepam for elderly patients should be cautious, usually starting at the lower end of the dosing range (seeDOSAGE AND ADMINISTRATION).
OXAZEPAM ADVERSE REACTIONS
OVERDOSAGE
Symptoms
Management
DOSAGE & ADMINISTRATION
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

OxazepamOxazepam CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!