Otic Care Antipyrine and Benzocaine
Otic Care Otic Solution Antipyrine, Benzocaine Rx Only
FULL PRESCRIBING INFORMATION: CONTENTS*
- OTIC CARE ANTIPYRINE AND BENZOCAINE DESCRIPTION:
- INACTIVE INGREDIENTS:
- CLINICAL PHARMACOLOGY:
- OTIC CARE ANTIPYRINE AND BENZOCAINE INDICATIONS AND USAGE:
- OTIC CARE ANTIPYRINE AND BENZOCAINE CONTRAINDICATIONS:
- PRECAUTIONS:
- OTIC CARE ANTIPYRINE AND BENZOCAINE ADVERSE REACTIONS:
- OTIC CARE ANTIPYRINE AND BENZOCAINE DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- WARNING:
- STORAGE:
- Image Label
FULL PRESCRIBING INFORMATION
DESCRIPTION:
TOPICAL ANESTHETIC AND ANALGESIC:
Otic Care Otic Solution
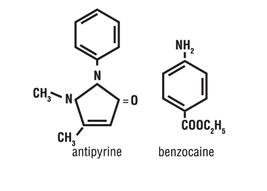
INACTIVE INGREDIENTS:
Acetic Acid, Aluminum Acetate, Aluminum Oxide, Glycerin, Policosanol.
CLINICAL PHARMACOLOGY:
Otic Care Otic Solution combines the properties of acetic acid, policosanol and aluminum acetate with the analgesic action of antipyrine and the local anesthetic action of benzocaine to relieve pressure, reduce inflammation and congestion, and alleviate pain and discomfort in acute otitis media. Does not blanch the tympanic membrane or mask the landmarks and therefore, does not distort the otoscopic picture.
INDICATIONS AND USAGE:
Acute otitis media of various etiologies:
- Rapid pain relief and reduction of inflammation in the congestive and serous stages of otitis media.
- Adjuvant therapy during systemic antibiotic administration.
- Facilitates the removal of excessive or impacted earwax.
CONTRAINDICATIONS:
- Hypersensitivity to any of the components or substances related to Otic Care Otic Solution.
- In case of tympanic membrane perforation or discharge.
PRECAUTIONS:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
No long-term studies in animals or humans have been conducted.
Pregnancy Category C:
Nursing Mothers:
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman. Consult your physician.
ADVERSE REACTIONS:
Call your doctor for medical advice about side effects. You may report suspected side effects to the FDA at 1-800-FDA-1088.
DOSAGE AND ADMINISTRATION:
Warm to body temperature (37°C or 98°F) by holding the bottle in the hand for a few minutes. To apply eardrops, wash your hands first. To avoid contamination do not touch the dropper or let it touch your ear or any surface. Instill Otic Care Otic Solution by tilting the affected ear upward, holding the dropper directly over the ear, and placing the prescribed number of drops into the ear canal. To help the drops roll into the ear of an adult, hold the earlobe up and back. In children, hold the earlobe down and back. Keep the head tilted about two minutes. Moisten a cotton pledget with Otic Care Otic Solution and insert into the opening of the ear canal. Repeat every one to two hours until pain and congestion is relieved.
Instill Otic Care Otic Solution three times daily for two or three days to help detach cerumen from wall of canal and facilitate removal.
Otic Care Otic Solution is useful for drying out the canal or relieving discomfort.
Otic Care Otic Solution
If swallowed or overdose is suspected, contact your local Poison Control Center or emergency room immediately. You may call US National Poison Hotline at 1-800-222-1222.
Note: Do not rinse dropper after use.
Replace dropper in bottle after each use. Hold dropper assembly by screw cap and, without compressing the rubber bulb, insert into drug container and screw down tightly.
Protect the solution from light and heat, and do not use if it is brown or contains a precipitate.
DISCARD THIS PRODUCT SIX MONTHS AFTER DROPPER IS FIRST PLACED IN THE DRUG SOLUTION.
HOW SUPPLIED:
14 mL of Otic Care Otic Solution ear drops are supplied in a white plastic bottle with separate dropper with screw cap attachment and package insert, NDC 59088-864-02.
WARNING:
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
STORAGE:
Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature]. Protect from freezing. Keep container tightly closed.
Image Label

Otic Care Antipyrine and BenzocaineAntipyrine, Benzocaine SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||