Oral Saline Laxative
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablespoon)
- Purpose
- Use
- Warnings
- Directions
- Oral Saline Laxative Other information
- Inactive ingredients
- Questions
- Package/Label Principal Display Panel
- Package Drug Facts 1
- Package Drug Facts 2
FULL PRESCRIBING INFORMATION
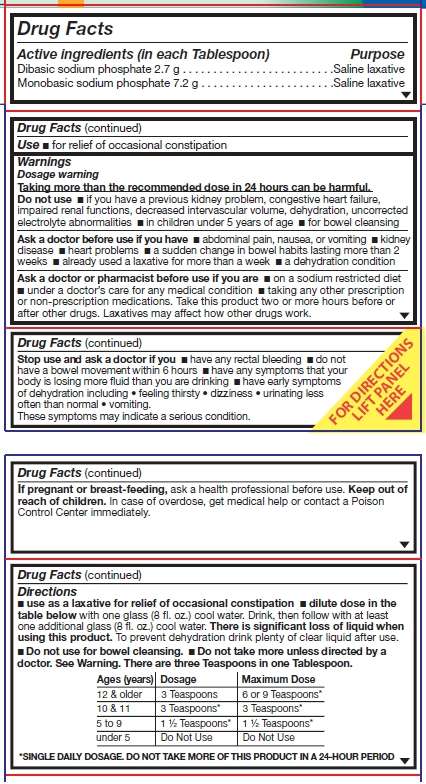
Active ingredient (in each tablespoon)
Dibasic sodium phosphate 2.7 g
Monobasic sodium phosphate 7.2 g
Purpose
Saline laxative
Use
- for relief of occasional constipation
Warnings
Dosage warning
Taking more than the recommended dose in 24 hours can be harmful.
Do not use
- if you have a previous kidney problem, congestive heart failure, impaired renal functions, decreased intervascular volume, dehydration, uncorrected electrolyte abnormalities
- in children under 5 years of age
- for bowel cleansing
Ask a doctor before use if you have
- abdominal pain, nausea, or vomiting
- kidney disease
- heart problems
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than a week
- a dehydration condition
Ask a doctor or pharmacist before use if you are
- on a sodium restricted diet
- under a doctor’s care for any medical condition
- taking any other prescription or non-prescription medications. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
Stop use and ask a doctor if you
- have any rectal bleeding
- do not have a bowel movement within 6 hours
- have any symptoms that your body is losing more fluid than you are drinking
- have early symptoms of dehydration including feeling thirsty, dizziness, urinating less often than normal, vomiting.
These symptoms may indicate a serious condition.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center immediately.
Directions
- use as a laxative for relief of occasional constipation
- dilute dose in the table below with one glass (8 fl. oz.) cool water. Drink, then follow with at least one additional glass (8 fl. oz.) cool water. There is significant loss of liquid when using this product. To prevent dehydration drink plenty of clear liquid after use.
- Do not use for bowel cleansing.
- Do not take more unless directed by a doctor. See Warning. There are three Teaspoons in one Tablespoon.
-
Ages (Years) Dosage Maximum Dose 12 & Older 3 Teaspoons 6 or 9 Teaspoons* 10 & 11 3 Teaspoons* 3 Teaspoons* 5 to 9 1 1/2 Teaspoons* 1 1/2 Teaspoons* under 5 Do Not Use Do Not Use *SINGLE DAILY DOSAGE. DO NOT TAKE MORE OF THIS PRODUCT IN A 24-HOUR PERIOD
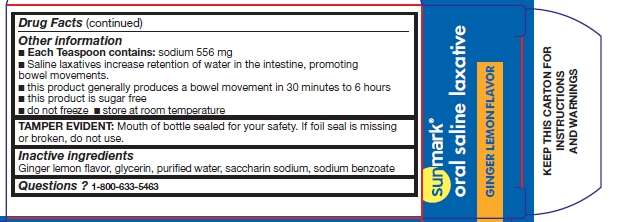
Oral Saline Laxative Other information
- Each Teaspoon contains: sodium 556 mg
- Saline laxatives increase retention of water in the intestine, promoting bowel movements.
- this product generally produces a bowel movement in 30 minutes to 6 hours
- this product is sugar free
- do not freeze
- store at room temperature
TAMPER EVIDENT: Mouth of bottle sealed for your safety. If foil seal is missing or broken, do not use.
Inactive ingredients
Ginger lemon flavor, glycerin, purified water, saccharin sodium, sodium benzoate
Questions
1-800-633-5463
Package/Label Principal Display Panel

sunmark
NDC 49348-111-12
Oral Saline Laxative
Sugar Free
For relief of occasional constipation
Single dose container
GINGER LEMON FLAVOR
1.5 FL OZ (45 mL)
Package Drug Facts 1
Package Drug Facts 2
Oral Saline Laxativedibasic sodium phosphate, monobasic sodium phosphate LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||