Oral-B Minute-Foam Orang-A-Tangy
Package.Label Principal Display Panel
FULL PRESCRIBING INFORMATION
MINUTE-FOAMTopical Fluoride Foaming Solution
(Acidulated Phosphate Fluoride Containing 1.23 % w/w Fluoride Ion)
FOR PROFESSIONAL USE ONLY
Active Ingredient: Acidulated Phosphate Fluoride (Sodium Fluoride and Hydrofluoric Acid) 1.23 % w/w fluoride ion.
DESCRIPTION: Oral-B Minute Foam is a flavored aqueous foaming solution of acidulated phosphate sodium fluoride with pH of 3.5 and a fluoride ion concentration of 1.23% w/w.
Inactive Ingredients: Purified water, poloxamer 407, isobutane, poloxamer 234, phosphoric acid, sodium saccharin, flavor.
CLINICAL PHARMACOLOGY: Acidulated Phosphate Fluoride inhibits caries formation by reducing enamel solubility and enhancing remineralization.
Uses
INDICATION AND USE: A topically-applied foaming solution to aid in the prevention of dental caries. CONTRAINDICATIONS: Do not use in patients with hypersensitivity to fluoride.
Do not use in patients with dysphagia. WARNINGS: DO NOT SWALLOW
Accidental ingestion of the usual treatment dose (approx 12.4 mg of fluoride) is not harmful. In the event more than the treatment dose is swallowed, administer calcium (e.g. milk) and get medical or contact a Poison control Center right away. One bottle of Minute Foam contained 1.95 grams of fluoride ion which could be lethal for children adults.
Keep out of the reach of infants and children under 12 years.
Pediatric patients under age 12 should be supervised during use of this product.
Avoid spraying towards open flame.
Contents under pressure. Do not puncture or incinerate. Do not expose to heat or store at temperatures over 120 F (49 C).
PRECAUTIONS: FOR PROFESSIONAL USE ONLY
Laboratory studies have indicated that repeated use of Acidulated Phosphate Fluoride products may dull porcelain and composite restorations.
Safety and effectiveness below age 6 have not been established. There have been no long term studies with this product to evaluate carcinogenic, mutagenic or impairment of fertility potential. Carcinogenesis, Mutagenesis, Impairment of Fertility:
No evidence of carcinogenicity was observed in female and male mice at doses ranging from 2.4 to 18.8 mg/kg sodium fluoride of body weight (3,4). Equivocal evidence of carcinogenicity was reported in male rats at doses ranging from 2.5 to 4.1 mg/kg fluoride, but no evidence of carcinogenicity was observed in female rates (3,4). In another study, no carcinogenicity was observed in rats treated with fluoride up to25 mg/kg of body weight (5). Overall, epidemiological studies do not show an association between fluoridated drinking water and increased cancer risks in humans (7).
Fluoride ion is not mutagenic in standard bacterial systems but has been associated with genetic aberrations in cultured human cells at doses much higher than expected for human exposure (6,8). Some in vivo studies report chromosomal aberrations in rodents while other studies using similar protocols report negative results (7).
Potential adverse reproductive effects of fluoride exposure in humans have not been adequately evaluated. Adverse reproductive effects of fluoride have been reported in animal studies, but at high concentrations sufficient to produce other manifestations of toxicity (9). Pregnancy:Teratogenic Effects: Pregnancy Category B. Fluoride readily crosses the placenta (7,9). Animal studies (rats and rabbits) have shown that fluoride is not a teratogen (10,12,13). Maternal exposure to 18 mg Fluoride/kg of body weight did not affect maternal body weight, litter size or fetal weight and did not increase frequency of skeletal or visceral malformations (10). There are no adequate and well-controlled studies in pregnant women. Several epidemiological studies show no increase in birth defects in areas with fluoridated water compared to areas with low fluoridated water (7). However, caution should be exercised when fluoride is administered to pregnant women. Nursing Mothers: Due to the relative insensitivity of human milk fluoride levels to changes in maternal fluoride intake, and due to the low concentrations of fluoride in human milk, fluoride supplementation during lactation would not be expected to significantly affect fluoride intake by the nursing infant (11). However, caution should be exercised when fluoride is administered to nursing women. Pediatric Use: The use of fluoride solutions, gels, and foams containing up to 1.23 % fluoride ion as caries preventives in pediatric patients aged 6 to 16 years is supported by clinical studies in students aged 6 to 12 years (1,2). Safety and effectiveness in pediatric patients below the age of 6 years has not been established. Please refer to CONTRAINDICATIONS and WARNINGS sections. Geriatric Use: No overall differences in safety or effectiveness have been observed between geriatric and younger patients. This drug is known to be substantially excreted by the kidney, therefore the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. ADVERSE REACTIONS: Developing teeth of children under age 6 may become permanently discolored if excessive amounts are repeatedly swallowed. The following adverse reactions are possible in individuals hypersensitive to fluoride: eczema, atopic dermatitis, urticaria, gastric distress, headache and weakness.OVERDOSAGE:
DOSAGE AND ADMINISTRATION:
HOW SUPPLIED:REFERENCES:
Minute Foam Label 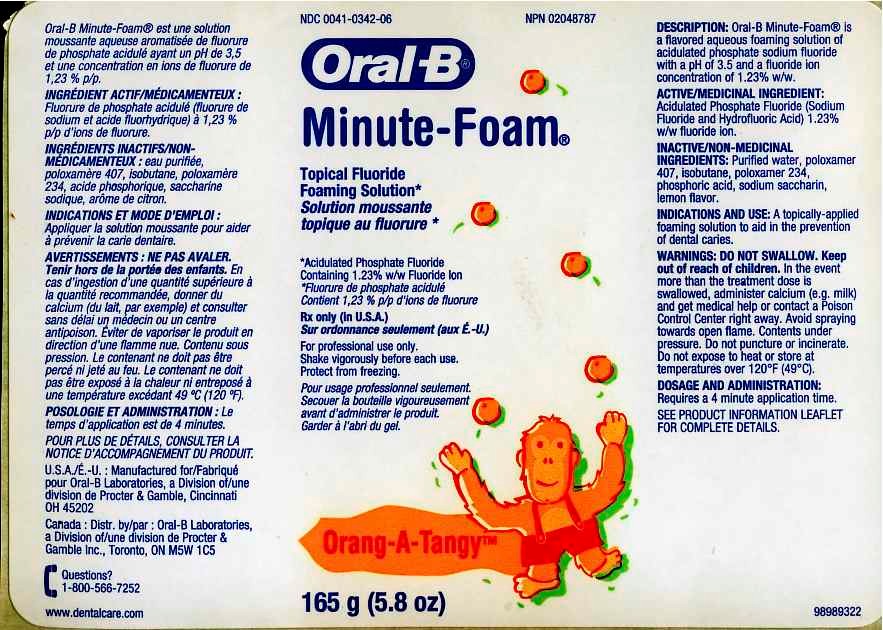
Oral-B Minute-Foam Orang-A-TangyAcidulated Phosphate Fluoride AEROSOL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!