Omega Pain Relieving
International Pharmaceuticals, Inc.
International Pharmaceuticals, Inc.
Omega Pain Relieving Liniment
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients Purpose
Camphor 4.0 percent
Menthol 3.33 percent
Methyl Salicylate 14.775 percent .....................................Counterirritant
Purpose
Uses
For the temporary relief of minor aches and pains of muscle and joints associated with:
Simple Backache
Arthritis
Strains
Warnings
For external use only. Avoid Contact with eyes.
If condition worsens, or if symptoms persist for more than
7 days or clear up and occur again within a few days,
discontinue use of this product and consult a doctor.
When used for arthritis, if pain persists for more than 10 days,
or redness is present, or in conditions affecting children under
12 years of age, consult a physician immediately.
Do Not
use otherwise than as directed.
apply to wounds or damaged skin.
bandage tightly.
Stop use and ask a doctor if
redness is present.
excessive irritation of the skin develops.
Directions
Shake well before using.
Adults and children 3 years of age and older: Apply to affected area not more than 3 to 4 times daily.
Children under 3 years of age: Consult a physician.
Other information
Store at 20 to 30 celsius (68 to 86 Fahrenheit)
Omega Pain Relieving Liniment
For external use only.
Net 4 fl oz (120 ml)
For your protection, this bottle is provided with a pilfer proof cap imprinted with the IPI
logo in red. Do not use if cap has been tampered with or is broken or destroyed.

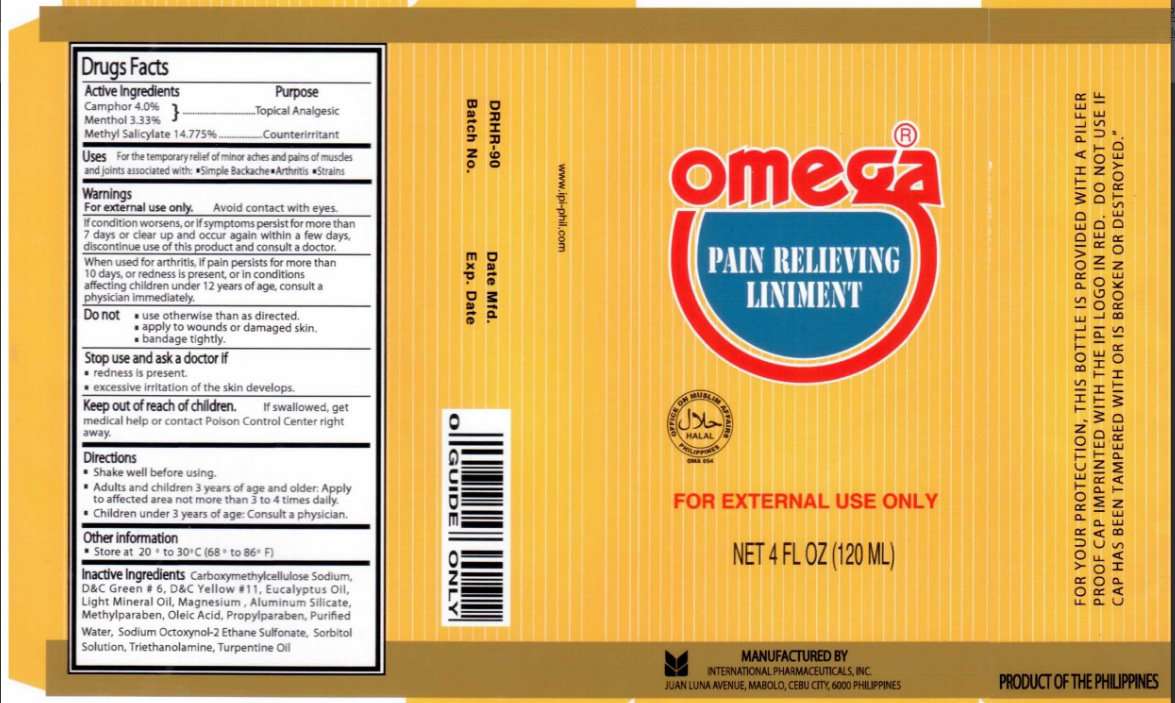
Manufactured By
International Pharmaceuticals, Inc.
Juan Luna Avenue, Mabolo,
Cebu City 6000 Philippines
Product Of The Philippines
Shake well before using
www.ipi-phil.com
Omega Pain RelievingCAMPHOR, MENTHOL, METHYL SALICYLATE LINIMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||