OHUI White Extreme Illuminating Pact No.10
LG Household and Healthcare, Inc.
LG Household and Healthcare, Inc.
Drug Fact
FULL PRESCRIBING INFORMATION
Active ingredient
Stop use if a rash or irritation develops and lasts.
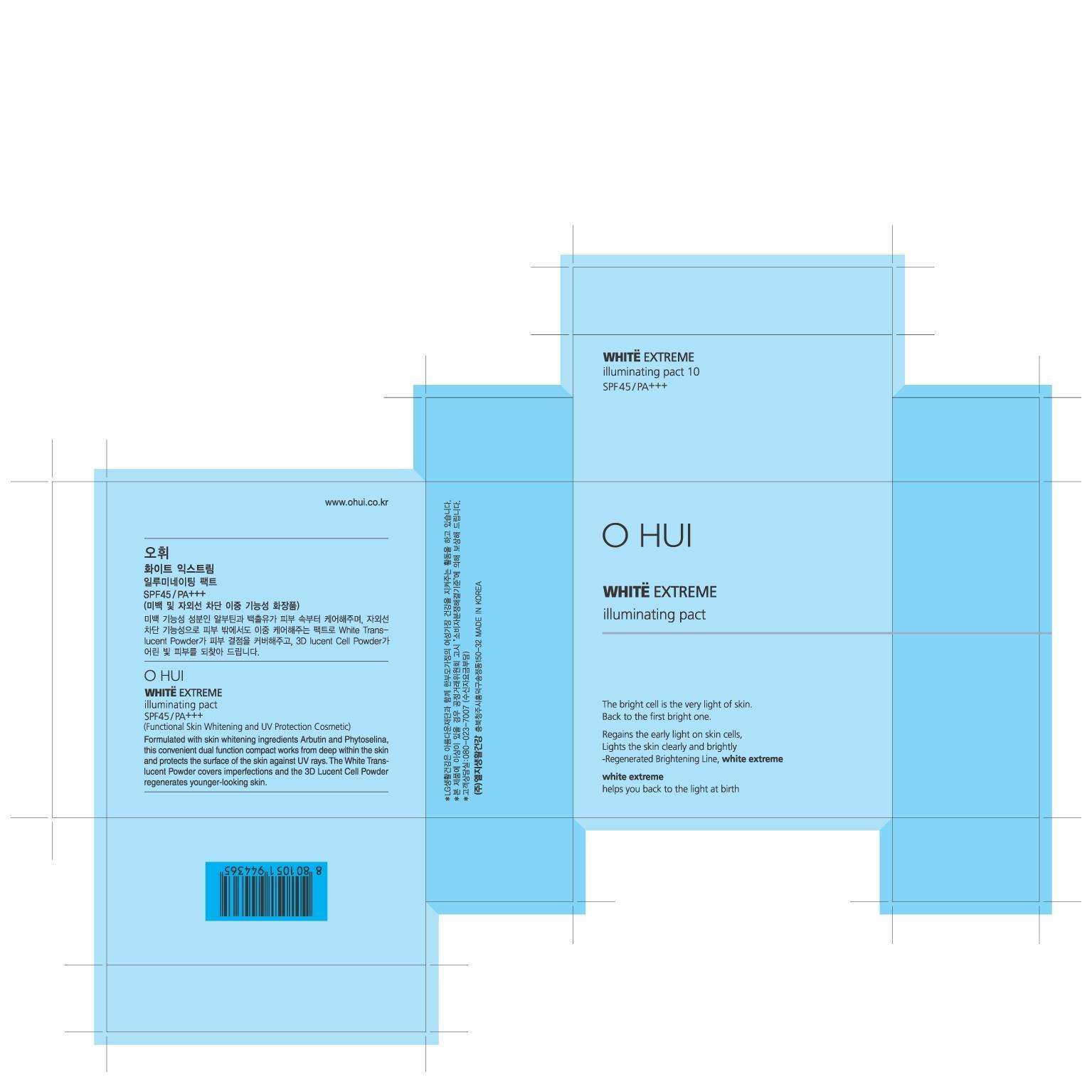
OHUI White Extreme Illuminating Pact No.10TITANIUM DIOXIDE, OCTINOXATE, ARBUTIN, ATRACTYLODES JAPONICA ROOT OIL POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!