OHUI Sun Science intensive sunblock cake EX
LG Household and Healthcare, Inc.
LG Household and Healthcare, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENT
ZINC OXIDE 10.528g/100g
OCTINOXATE 7g/100g
TITANIUM DIOXIDE 4g/100g
BEMOTRIZINOL 1.5g/100g
WARNINGS AND PRECAUTIONS
For external use only.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
STOP USE
Stop use if a rash or irritation develops and lasts.
WHEN USING
Keep out of eyes. Rinse with water to remove.
OHUI
Sun Science
Intensive Sunblock Cake EX
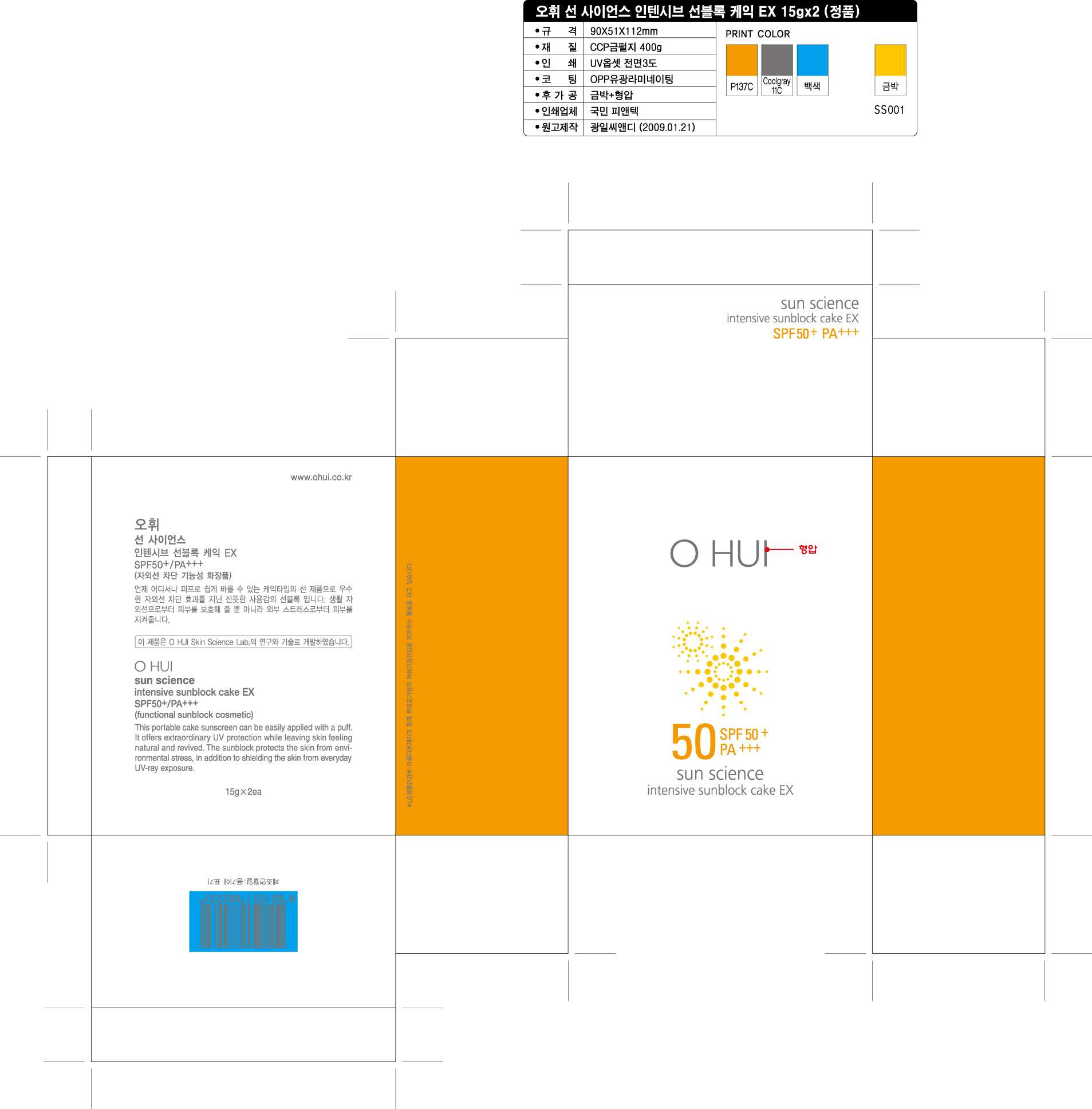
OHUI Sun Science intensive sunblock cake EXZINC OXIDE, OCTINOXATE, TITANIUM DIOXIDE, BEMOTRIZINOL CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!