Nibec Co., Ltd
Nibec Co., Ltd
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Purpose
Uses
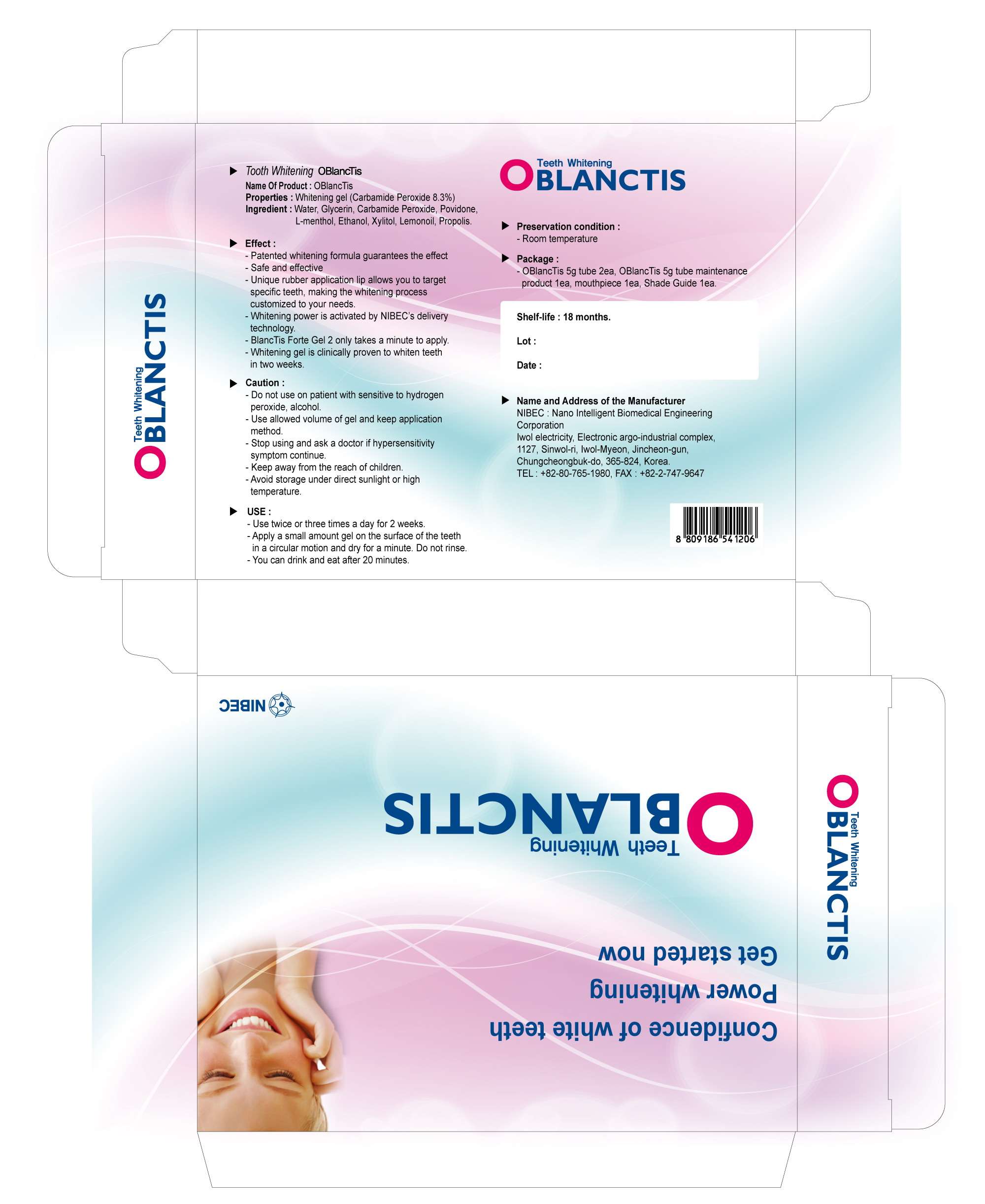
O BlancTis
alcohol GEL, DENTIFRICE
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:47649-6001 |
|
Route of Administration
|
DENTAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
ALCOHOL ALCOHOL |
|
50 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:47649-6001-1 |
5 in 1 TUBE |
|
|
|
2 |
NDC:47649-6001-2 |
3 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2012-02-07 |
|
|

