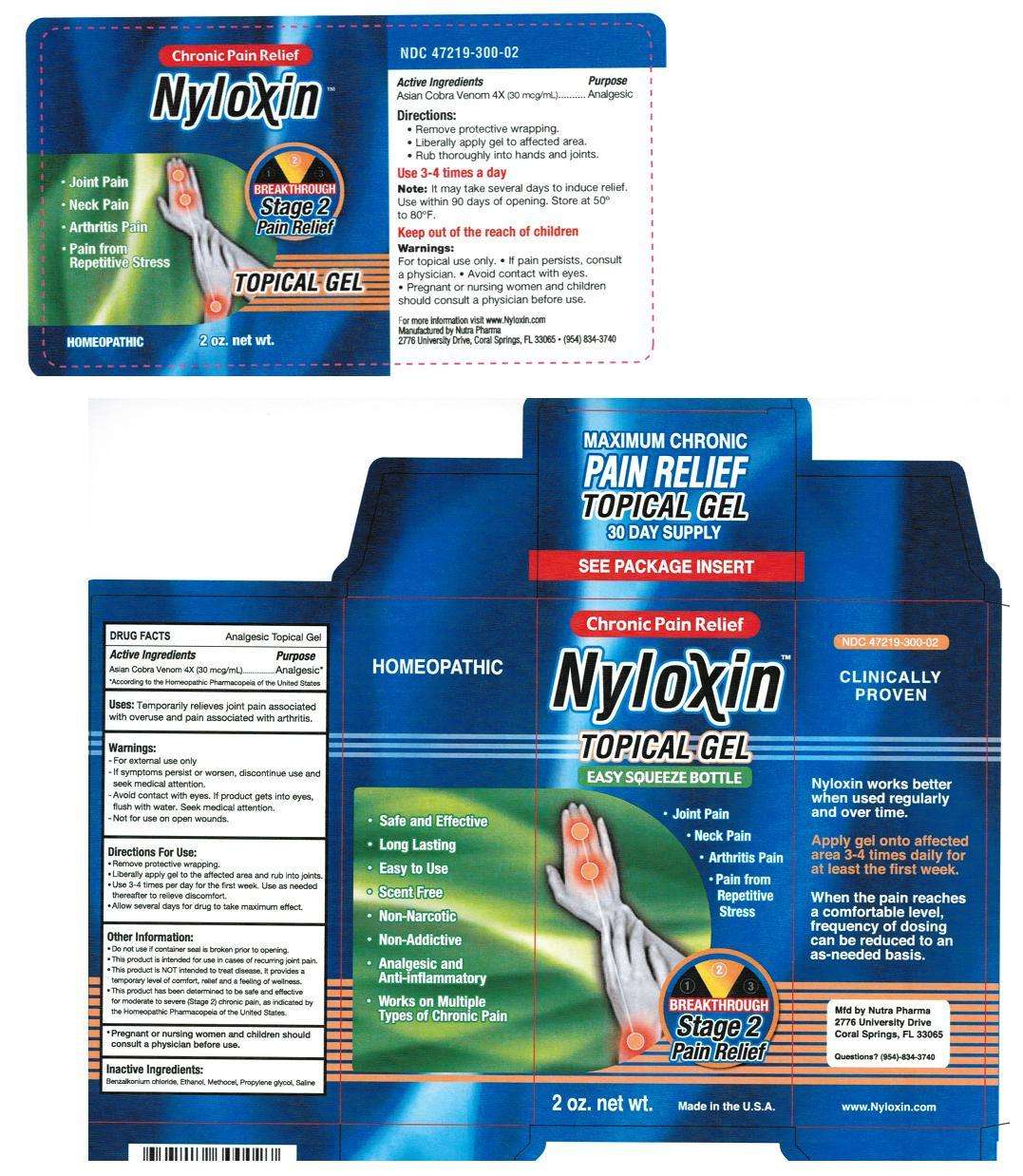
Nyloxin
Nyloxin Topical Gel
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Nyloxin Uses:
- Warnings:
- Directions for Use:
- Other Information:
- Inactive Ingredients:
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredients
Asian Cobra Venom 4x (30 mcg/mL)
Purpose
Analgesic*
___________________________________________________________
*According to the Homeopathic Pharmacopeia of the United States
Uses:
Temporarily relieves joint pain associated with overuse and pain associated with arthritis.
Warnings:
- For external use only.
- If symptoms persist or worsen, discontinue use and seek medical attention.
- Avoid contact with eyes. If product gets into eyes flush with water. Seek Medical attention.
- Not for use on open wounds.
Keep out of the reach of children
Directions for Use:
- Remove protective wrapping
- Liberally apply gel to the affected area and rub into joints.
- Use 3-4 time per day for the first week. Use as needed thereafter to relieve discomfort.
- Allow several days for drug to take maximum effect.
Other Information:
- Do not use if container seal is broken prior to opening.
- This product is intended for use in cases of recurring joint pain.
- This product is NOT intended to treat disease, it provides a temporary level of comfort, relief and a feeling of wellness.
- This product has been determined to be safe and effective for moderate to severe (Stage 2) chronic pain, as indicated by the Homeopathic Pharmacopeia of the United States.
- Pregnant or nursing women and children should consult a physician before use.
Inactive Ingredients:
Benzalkonium chloride, Ethanol, Methocel, Propylene glycol, Saline.
For more information visit: www.Nyloxin.com
Manufactured by NutraPharma
2775 University Drive, Coral Springs, FL 33065 (954) 834-3740
Principal Display Panel
NDC 47219-300-02
Nyloxin Topical Gel
Easy Squeeze Bottle
Maximum Chronic Pain Relief
Topical Gel
30 DAY SUPPLY
NyloxinNAJA NAJA VENOM GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||