NU SKIN
NU SKIN ageLOC RADIANT DAY SPF 22
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- NU SKIN Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions
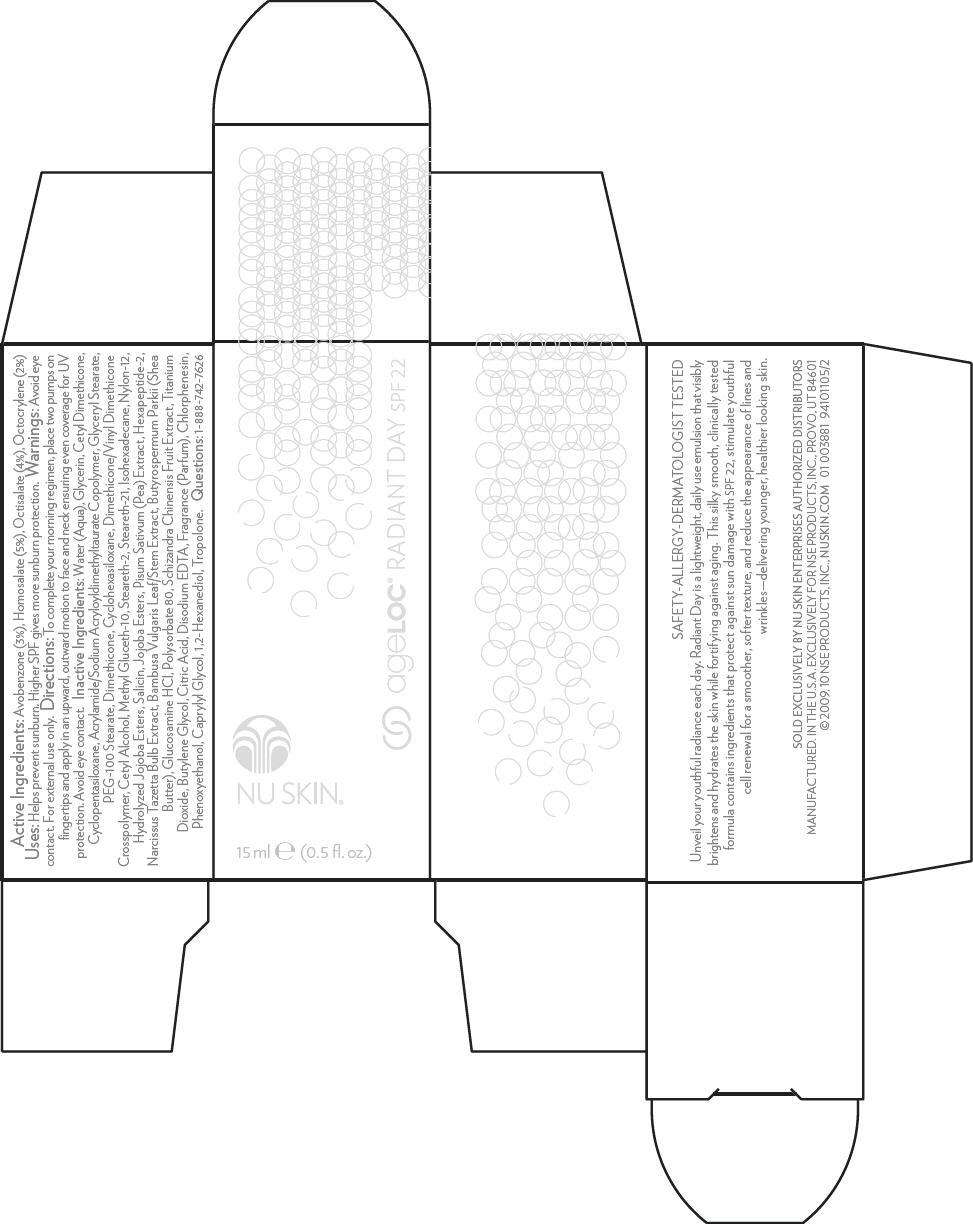
- PRINCIPAL DISPLAY PANEL - 15 ml Carton
FULL PRESCRIBING INFORMATION
Active Ingredients
Avobenzone (3%), Homosalate (5%), Octisalate (4%), Octocrylene (2%)
NU SKIN Uses
Helps prevent sunburn. Higher SPF gives more sunburn protection.
Warnings
Avoid eye contact. For external use only.
Directions
To complete your morning regimen, place two pumps on fingertips and apply in an upward, outward motion to face and neck ensuring even coverage for UV protection. Avoid eye contact.
Inactive Ingredients
Water (Aqua), Glycerin, Cetyl Dimethicone, Cyclopentasiloxane, Acrylamide/Sodium Acryloyldimethyltaurate Copolymer, Glyceryl Stearate, PEG-100 Stearate, Dimethicone, Cyclohexasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Cetyl Alcohol, Methyl Gluceth-10, Steareth-2, Steareth-21, Isohexadecane, Nylon-12, Hydrolyzed Jojoba Esters, Salicin, Jojoba Esters, Pisum Sativum (Pea) Extract, Hexapeptide-2, Narcissus Tazetta Bulb Extract, Bambusa Vulgaris Leaf/Stem Extract, Butyrospermum Parkii (Shea Butter), Glucosamine HCl, Polysorbate 80, Schizandra Chinensis Fruit Extract, Titanium Dioxide, Butylene Glycol, Citric Acid, Disodium EDTA, Fragrance (Parfum), Chlorphenesin, Phenoxyethanol, Caprylyl Glycol, 1,2-Hexanediol, Tropolone.
Questions
1-888-742-7626
PRINCIPAL DISPLAY PANEL - 15 ml Carton
NU SKIN®
15 ml e (0.5 fl. oz.)
ageLOC ® RADIANT DAY SPF 22
NU SKINAvobenzone, Homosalate, Octisalate, and Octocrylene LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||