NOURISHING hydrator
NOURISHING HYDRATOR SPF 15 1.7 fl oz (50mL)
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active Ingredients
Avobenzone 2%
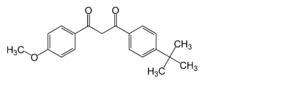
Octinoxate 7.5%
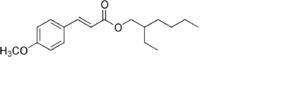
Oxybenzone 3.5%

Purpose
Sunscreen
Uses:
- Helps prevent sunburn
Warnings:
- For External use only
- Avoid Contact with Eyes.
- Stop use and ask a doctor if irritation or rash occurs
- Keep Out of Reach of Children. If swallowed get medical help or contact a Poison Control Center immediately.
Directions
- Each morning massage a thin layer over face, neck and chest.
Inactive Ingredients:
Water, Cyclomethicone, Dimethicone Crosspolymer-3, Pentylene Glycol, Aminobutyric Acid, Glycerin, Sodium Dihydroxycetyl Phosphate, Cholesterol, Dimethicone, PEG-40 Stearate, Squalane, Butylene Glycol, Phenoxyethanol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Glycine Soja (Soybean) Seed Extract, Carbomer, Cetearyl Alcohol, Olea Europaea (Olive) Fruit Oil, Polysorbate 60, Vitis Vinifera (Grape) Seed Oil, Hypnea Musciformis Extract, Alpha-Arbutin, Caprylyl Glycol, Hydrolyzed Algin, Potassium Sorbate, Tetrasodium EDTA, Xanthan Gum, Chlorella Vulgaris Extract, Chlorphenesin, Palmitoyl Oligopeptide, Palmitoyl Tetrapeptide-7, Polysorbate 20, Sea Water, Sargassum Filipendula Extract, Hydrogenated Palm Glycerides, Algin, Avena Sativa (Oat) Kernel Extract, Ceramide 2, Retinyl Palmitate, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Sodium Citrate, Sorbitol, Atelocollagen, Gellidiela Acerosa Extract, Evodia Rutaecarpa Fruit Extract, Sodium Hydroxide, Serine, Sodium Hyaluronate, Ubiquinone
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Nourishing Hydrator 50mL Can Label
NDC 76082-210-50
ELAYDA
Professional Skincare
NOURISHING hydrator
UVA/UVB protection with antioxidant
SPF 15
1.7 fl oz (50mL)
