Nortriptyline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- NORTRIPTYLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- NORTRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- PEDIATRIC USE
- GERIATRIC USE
- NORTRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL MEDGUIDE
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
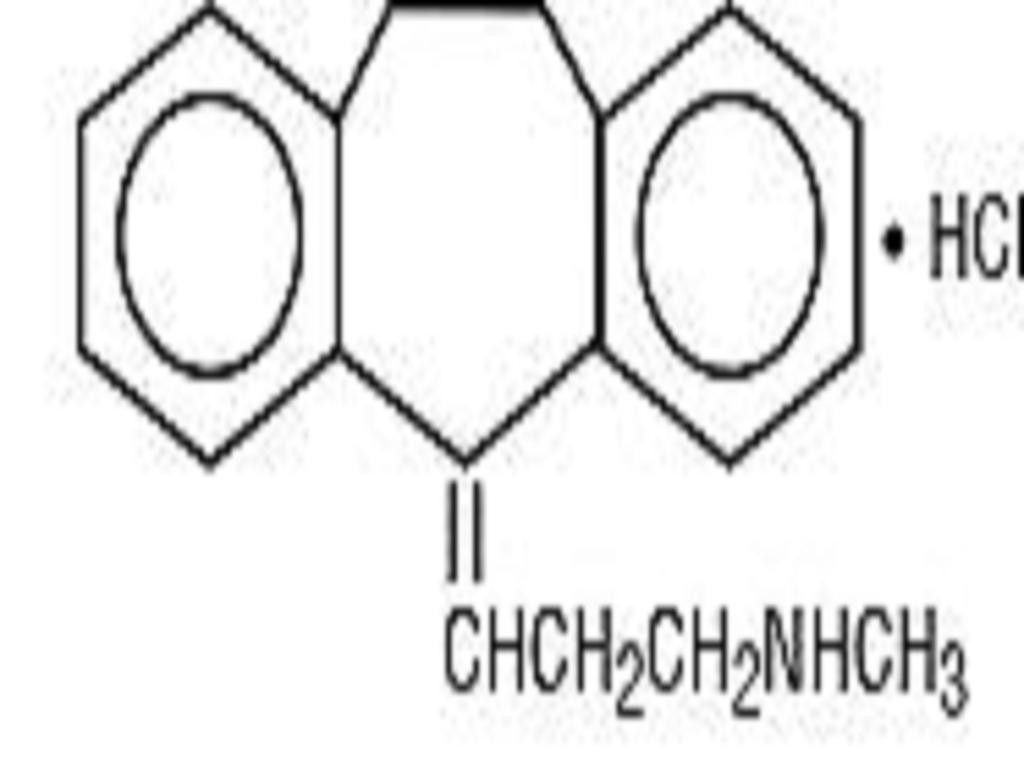
NORTRIPTYLINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
NORTRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskScreening Patients for Bipolar Disorder
Use in Pregnancy
PRECAUTIONS
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
DRUG INTERACTIONS
Drugs Metabolized by P450 2D6
PEDIATRIC USE
GERIATRIC USE
NORTRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
Manifestations
Management
General
Gastrointestinal Decontamination
Cardiovascular
CNS
Psychiatric Follow-up
Pediatric Management
DOSAGE & ADMINISTRATION
Usual Adult Dose
Elderly and Adolescent Patients
HOW SUPPLIED
SPL MEDGUIDE
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
-
● 1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
-
● thoughts about suicide or dying
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● What else do I need to know about antidepressant medicines?
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
-
● Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
● This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
INACTIVE INGREDIENT
STARCH, CORND&C YELLOW NO. 10
ALUMINUM OXIDE
FD&C BLUE NO. 1
FD&C BLUE NO. 2
FD&C RED NO. 40
GELATIN
FERROSOFERRIC OXIDE
METHYLPARABEN
PROPYLENE GLYCOL
PROPYLPARABEN
SHELLAC
SODIUM LAURYL SULFATE
TITANIUM DIOXIDE
FD&C YELLOW NO. 6
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Nortriptyline HydrochlorideNortriptyline Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!