Nizatidine
Nizatidine Insert & Label
FULL PRESCRIBING INFORMATION
Nizatidine USP is a histamine H2-receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N’-methyl-2-nitro-1,1-ethenediamine.
The structural formula is as follows:
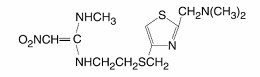
Nizatidine has the molecular formula C12H21N5O2S2 representing a molecular weight of 331.46. It is an off-white to buff crystalline solid that is soluble in water. Nizatidine has a bitter taste and mild sulfur-like odor.
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H2-receptors, particularly those in the gastric parietal cells.
Nizatidine significantly inhibited nocturnal gastric acid secretion for up to 12 hours. Nizatidine also significantly inhibited gastric acid secretion stimulated by food, caffeine, betazole, and pentagastrin .
Uses
Nizatidine is indicated for up to 8 weeks for the treatment of active duodenal ulcer. In most patients, the ulcer will heal within 4 weeks.
Nizatidine is indicated for maintenance therapy for duodenal ulcer patients, at a reduced dosage of 150 mg h.s. after healing of an active duodenal ulcer. The consequences of continuous therapy with nizatidine for longer than 1 year are not known.
Nizatidine is indicated for up to 12 weeks for the treatment of endoscopically diagnosed esophagitis, including erosive and ulcerative esophagitis, and associated heartburn due to GERD.
Nizatidine is indicated for up to 8 weeks for the treatment of active benign gastric ulcer. Before initiating therapy, care should be taken to exclude the possibility of malignant gastric ulceration.
Nizatidine is contraindicated in patients with known hypersensitivity to the drug. Because cross sensitivity in this class of compounds has been observed, H2-receptor antagonists, including nizatidine, should not be administered to patients with a history of hypersensitivity to other H2-receptor antagonists.
GeneralSymptomatic response to nizatidine therapy does not preclude the presence of gastric malignancy.
Because nizatidine is excreted primarily by the kidney, dosage should be reduced in patients with moderate to severe renal insufficiency (see DOSAGE AND ADMINISTRATION).
Pharmacokinetic studies in patients with hepatorenal syndrome have not been done. Part of the dose of nizatidine is metabolized in the liver. In patients with normal renal function and uncomplicated hepatic dysfunction, the disposition of nizatidine is similar to that in normal subjects.
Laboratory TestsFalse-positive tests for urobilinogen with Multistix® may occur during therapy with nizatidine.
Drug InteractionsNo interactions have been observed between nizatidine and theophylline, chlordiazepoxide, lorazepam, lidocaine, phenytoin, and warfarin. Nizatidine does not inhibit the cytochrome P-450-linked drug-metabolizing enzyme system; therefore, drug interactions mediated by inhibition of hepatic metabolism are not expected to occur. In patients given very high doses (3,900 mg) of aspirin daily, increases in serum salicylate levels were seen when nizatidine, 150 mg b.i.d., was administered concurrently.
Carcinogenesis, Mutagenesis, Impairment of FertilityA 2-year oral carcinogenicity study in rats with doses as high as 500 mg/kg/day (about 13 times the recommended human dose based on body surface area) showed no evidence of a carcinogenic effect. There was a dose-related increase in the density of enterochromaffin-like (ECL) cells in the gastric oxyntic mucosa. In a 2-year study in mice, there was no evidence of a carcinogenic effect in male mice; although hyperplastic nodules of the liver were increased in the high-dose males as compared with placebo. Female mice given the high dose of nizatidine (2,000 mg/kg/day, about 27 times the recommended human dose based on body surface area) showed marginally statistically significant increases in hepatic carcinoma and hepatic nodular hyperplasia with no numerical increase seen in any of the other dose groups. The rate of hepatic carcinoma in the high-dose animals was within the historical control limits seen for the strain of mice used. The female mice were given a dose larger than the maximum tolerated dose, as indicated by excessive (30%) weight decrement as compared with concurrent controls and evidence of mild liver injury (transaminase elevations). The occurrence of a marginal finding at high dose only in animals given an excessive and somewhat hepatotoxic dose, with no evidence of a carcinogenic effect in rats, male mice, and female mice (given up to 360 mg/kg/day, about 5 times the recommended human dose based on body surface aea), and a negative mutagenicity battery are not considered evidence of a carcinogenic potential for nizatidine.
Nizatidine was not mutagenic in a battery of tests performed to evaluate its potential genetic toxicity, including bacterial mutation tests, unscheduled DNA synthesis, sister chromatid exchange, mouse lymphoma assay, chromosome aberration tests, and a micronucleus test.
In a 2-generation, perinatal and postnatal fertility study in rats, doses of nizatidine up to 650 mg/kg/day (about 17.5 times the recommended human dose based on body surface area) produced no adverse effects on the reproductive performance of parental animals or their progeny.
PregnancyTeratogenic Effects–Pregnancy Category B: Oral reproduction studies in pregnant rats at doses up to 1500 mg/kg/day (about 40.5 times the recommended human dose based on body surface area) and in pregnant rabbits at doses up to 275 mg/kg/day (about 14.6 times the recommended human dose based on body surface area) have revealed no evidence of impaired fertility or harm to the fetus due to nizatidine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing MothersStudies conducted in lactating women have shown that 0.1% of the administered oral dose of nizatidine is secreted in human milk in proportion to plasma concentrations. Because of the growth depression in pups reared by lactating rats treated with nizatidine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric UseSafety and effectiveness in pediatric patients have not been established.
Geriatric UseOf the 955 patients in clinical studies who were treated with nizatidine, 337 (35.3%) were 65 and older. No overall differences in safety or effectiveness were observed between these and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see DOSAGE AND ADMINISTRATION).
Worldwide, controlled clinical trials of nizatidine included over 6,000 patients given nizatidine in studies of varying durations. Placebo-controlled trials in the United States and Canada included over 2,600 patients given nizatidine and over 1,700 given placebo. Among the adverse events in these placebo-controlled trials, anemia (0.2% vs 0%) and urticaria (0.5% vs 0.1%) were significantly more common in the nizatidine group.
Table 5 lists adverse events that occurred at a frequency of 1% or more among nizatidine-treated patients who participated in placebo-controlled trials. The cited figures provide some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence rate in the population studied.
A variety of less common events were also reported; it was not possible to determine whether these were caused by nizatidine.
Hepatic: Hepatocellular injury, evidenced by elevated liver enzyme tests (SGOT [AST], SGPT [ALT], or alkaline phosphatase), occurred in some patients and was possibly or probably related to nizatidine. In some cases there was marked elevation of SGOT, SGPT enzymes (greater than 500 IU/L) and, in a single instance, SGPT was greater than 2,000 IU/L. The overall rate of occurrences of elevated liver enzymes and elevations to 3 times the upper limit of normal, however, did not significantly differ from the rate of liver enzyme abnormalities in placebo-treated patients. All abnormalities were reversible after discontinuation of nizatidine. Since market introduction, hepatitis and jaundice have been reported. Rare cases of cholestatic or mixed hepatocellular and cholestatic injury with jaundice have been reported with reversal of the abnormalities after discontinuation of nizatidine.
Cardiovascular: In clinical pharmacology studies, short episodes of asymptomatic ventricular tachycardia occurred in 2 individuals administered nizatidine and in 3 untreated subjects.
CNS: Rare cases of reversible mental confusion have been reported.
Endocrine: Clinical pharmacology studies and controlled clinical trials showed no evidence of antiandrogenic activity due to nizatidine. Impotence and decreased libido were reported with similar frequency by patients who received nizatidine and by those given placebo. Rare reports of gynecomastia occurred.
Hematologic: Anemia was reported significantly more frequently in nizatidine- than in placebo-treated patients. Fatal thrombocytopenia was reported in a patient who was treated with nizatidine and another H2-receptor antagonist. On previous occasions, this patient had experienced thrombocytopenia while taking other drugs. Rare cases of thrombocytopenic purpura have been reported.
Integumental: Sweating and urticaria were reported significantly more frequently in nizatidine- than in placebo-treated patients. Rash and exfoliative dermatitis were also reported. Vasculitis has been reported rarely.
Hypersensitivity: As with other H2-receptor antagonists, rare cases of anaphylaxis following administration of nizatidine have been reported. Rare episodes of hypersensitivity reactions (e.g., bronchospasm, laryngeal edema, rash, and eosinophilia) have been reported.
Body as a Whole: Serum sickness-like reactions have occurred rarely in conjunction with nizatidine use.
Genitourinary: Reports of impotence have occurred.
Other: Hyperuricemia unassociated with gout or nephrolithiasis was reported. Eosinophilia, fever, and nausea related to nizatidine administration have been reported.
The recommended oral dosage for adults is 300 mg once daily at bedtime. An alternative dosage regimen is 150 mg twice daily.
The recommended oral dosage for adults is 150 mg once daily at bedtime.
The recommended oral dosage in adults for the treatment of erosions, ulcerations, and associated heartburn is 150 mg twice daily.
The recommended oral dosage is 300 mg given either as 150 mg twice daily or 300 mg once daily at bedtime. Prior to treatment, care should be taken to exclude the possibility of malignant gastric ulceration.
The dose for patients with renal dysfunction should be reduced as follows:
Some elderly patients may have creatinine clearances of less than 50 mL/min, and, based on pharmacokinetic data in patients with renal impairment, the dose for such patients should be reduced accordingly. The clinical effects of this dosage reduction in patients with renal failure have not been evaluated.
Nizatidine Capsules USP, 150 mg are available as white opaque body and yellow opaque cap, imprinted “ 150” on the body and cap in black ink. They are available in bottles of 60 and 500.
Nizatidine Capsules USP, 300 mg are available as white opaque body and peach opaque cap, imprinted “ 300” on the body and cap in black ink. They are available in bottles of 30 and 100.
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Sandoz, Inc.
Princeton, NJ 08540

Nizatidinenizatidine CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||