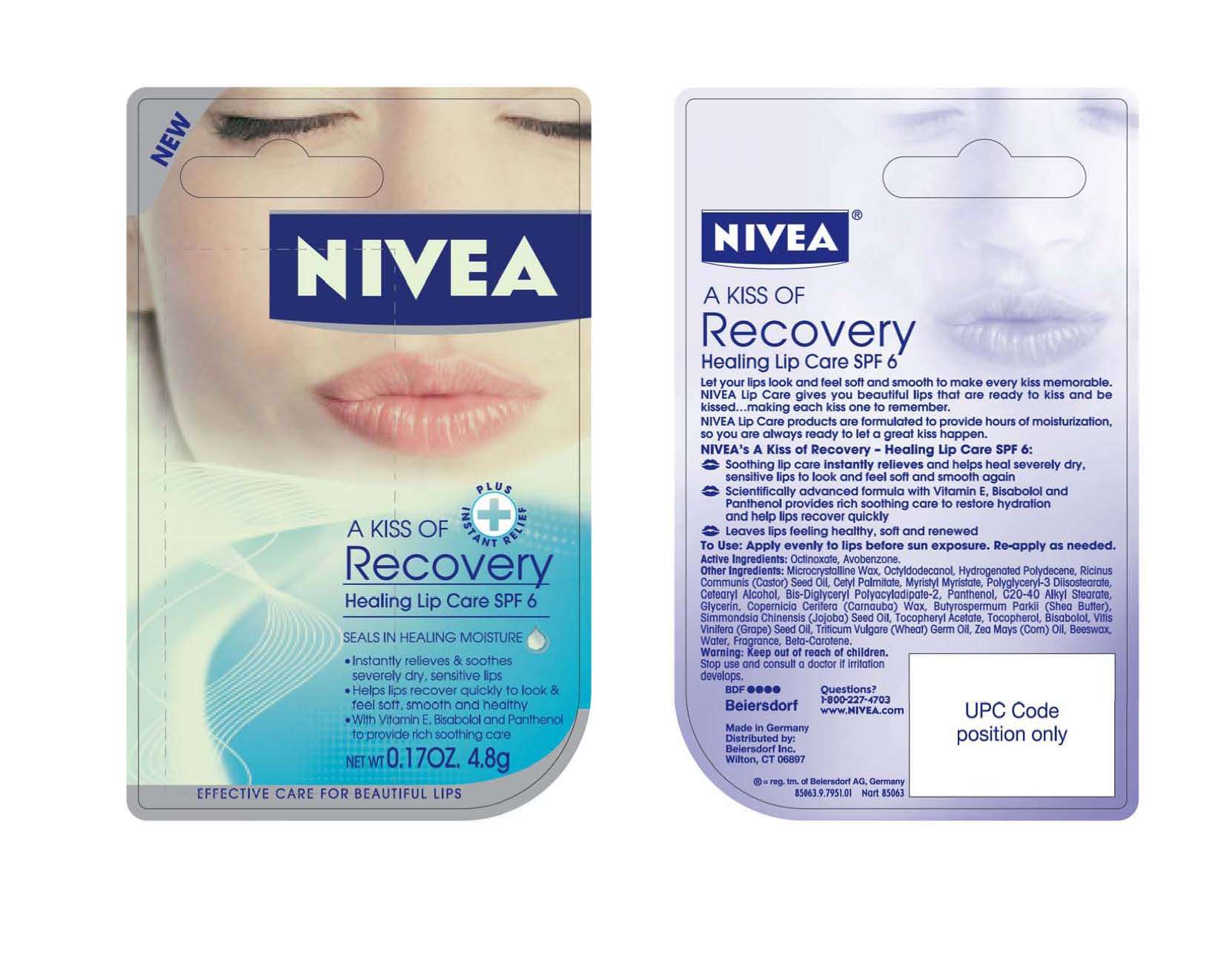
Nivea a Kiss of recovery
Nivea A Kiss of Recovery Healing Lip Care
FULL PRESCRIBING INFORMATION
Let your lips look and feel soft and smooth to make every kiss memorable. NIVEA Lip Care gives you beautiful lips that are ready to kiss and be kissed...making each kiss one to remember.
NIVEA's A Kiss of Recovery-Healing Lip Care SPF 6:
- Soothing lip care instantly relieves and hleps heal severely dry, sensitive lips to look and feel soft and smooth again
- Scientifically advanced formula with Vitamin E, Bisobalol and Panthenol provides rich soothing care to restore hydration and help lips recover quickly
- Leaves lips feeling healthy, soft and renewed
Active ingredient
Active Ingredients: Octinoxate, Avobenzone
Warning: Keep out of reach of children.
Stop use and consult a doctor if irritation develops.
Questions
18002274703
Uses
To Use: Apply evenly to lips before sun exposure. Re-apply as needed.
Other Ingredients: Microcrystalline Wax, Octyldodecanol, Hydrogenated Polydecene, Ricinus Communis (Castor) Seed Oil, Cetyl Palmitate,Myristyl Myristate, Polyglyceryl-3 Diisostearate, Cetearly Alcohol, Bis-Diglyceryl Polyacyladipate-2, Panthenol, C20-40 Alkyl Stearate, Glycerin, Copernicia Cerifera (Carnauba) Wax, Butyrospermum Parkii (Shea Butter), Simmondsia Chinensis (Jojoba) Seed Oil, Tocopheryl Acetate, Bisabolol, Vitis Vinifera (Grape) Seed Oil, Triticum Vulgare (Wheat) Germ Oil, Zea Mays (Corn) Oil, Beeswax, Water, Fragrance, Beta-Carotene.
Nivea a Kiss of recoveryoctinoxate, avobenzone STICK
| ||||||||||||||||||||||||||||||||||||||||||||||||||||