Nivea A Kiss of Recovery Medicated Lip Care
NIVEA A Kiss of Recovery Medicated Lip Care
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
Dimethicone 1.2%
Avobenzone 1.0%
Octinoxate 6.7%
Purpose
Purpose
Skin Protectant
Sunscreen
Uses
Uses
• temporarily protects and helps relieve chapped or cracked lips
• helps prevent sunburn
Warnings
Skin Cancer/Skin Aging Alert; Spending time in the sun increases your risk of
skin cancer and early skin aging. This product has been shown only to help prevent
sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product avoid contact with eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a
Poison Control Center right away
Directions
• apply liberally before sun exposure
• reapply as needed
• children under 6 months of age: ask a doctor
Inactive ingredients
Microcrystalline Wax, Octyldodecanol, Hydrogenated Polydecene, Ricinus Communis (Castor) Seed Oil, Cetyl Palmitate,
Myristyl Myristate, Polyglyceryl-3 Diisostearate, Cetearyl Alcohol, Bis-Diglyceryl Polyacyladipate-2, Panthenol,
C20-40 Alkyl Stearate, Glycerin, Copernicia Cerifera (Carnauba) Wax, Butyrospermum Parkii (Shea) Butter,
Simmondsia Chinensis (Jojoba) Seed Oil, Oenothera Biennis (Evening Primrose) Oil, Ascorbyl Palmitate, Tocopherol,
Tocopheryl Acetate, Menthol, Beeswax, Water, BHT, Fragrance, Iron Oxides
Questions? 1-800-227-4703
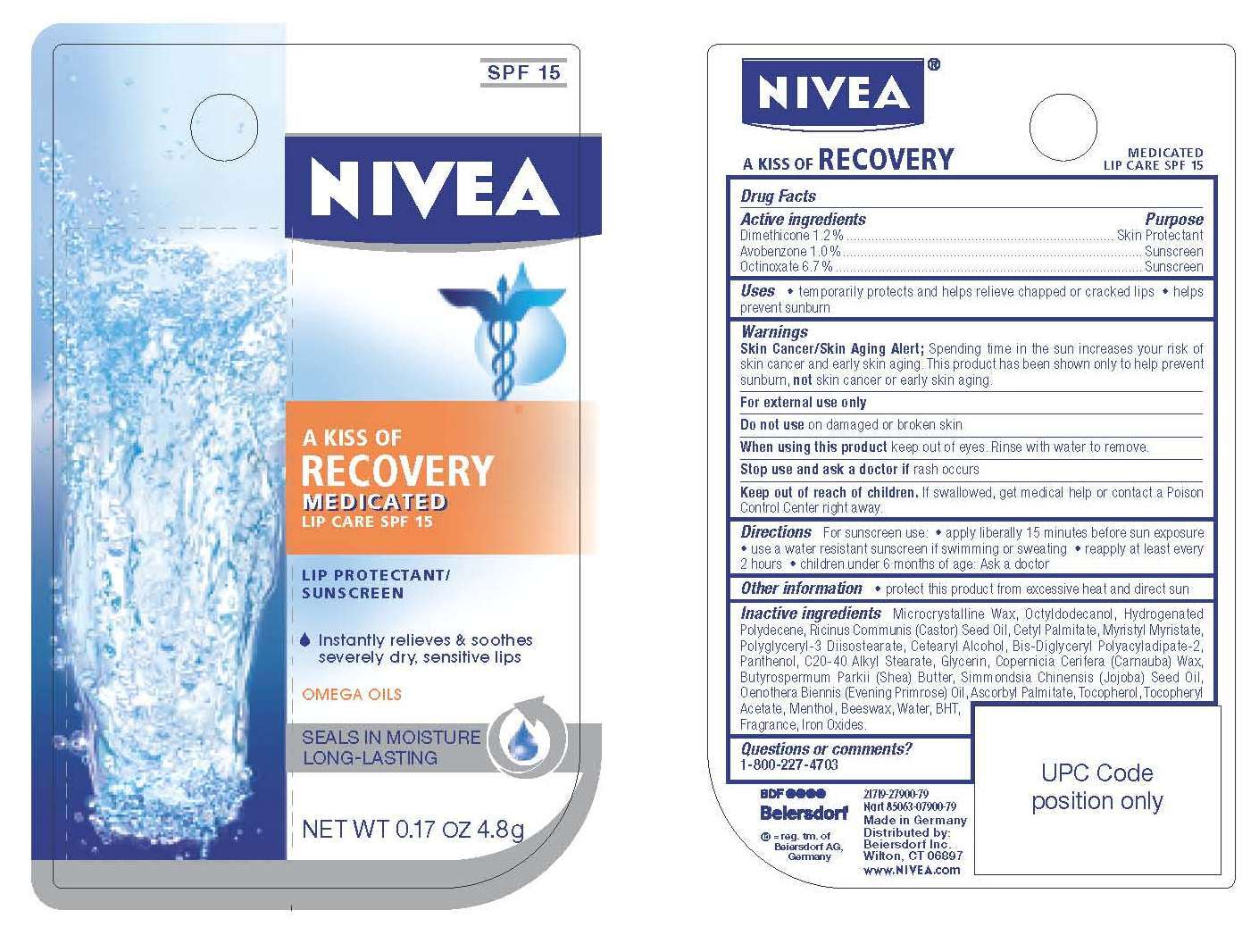
SPF 15
NIVEA
A KISS OF RECOVERY
MEDICATED LIP CARE SPF 15
LIP PROTECTANT / SUNSCREEN
Instantly relieves and soothes
severely dry, sensitive lips
OMEGA OILS
SEALS IN MOISTURE
LONG-LASTING
Nivea A Kiss of Recovery Medicated Lip CareDimethicone, Avobenzone, Octinoxate STICK
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||