Nitrostat
FULL PRESCRIBING INFORMATION: CONTENTS*
- NITROSTAT DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- NITROSTAT CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- NITROSTAT ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
NITROSTAT DESCRIPTION
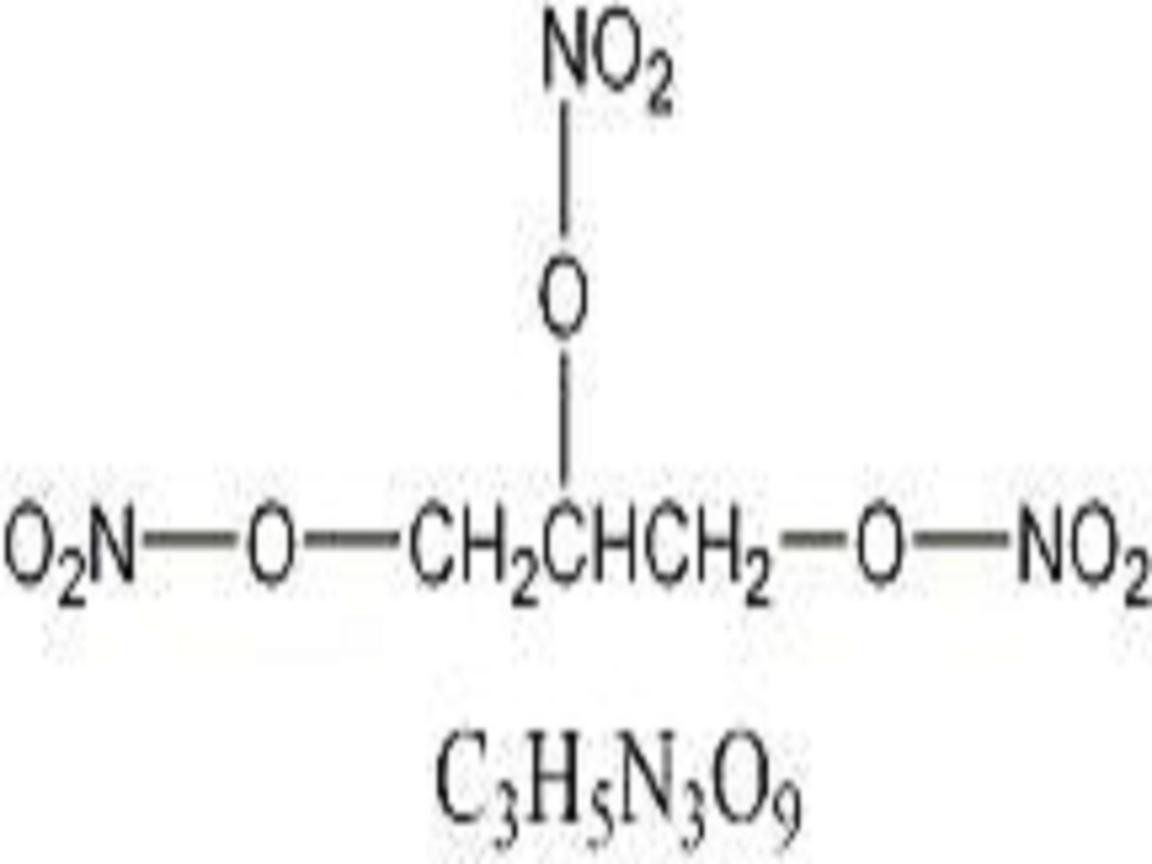
CLINICAL PHARMACOLOGY
Mechanism of Action
Pharmacodynamics
Pharmacokinetics and Drug Metabolism Absorption
Mean Nitroglycerin (SD) Values20.3 mg10.6 mgParameterNITROSTAT TabletsNITROSTAT Tablets
Distribution
Metabolism
Elimination
INDICATIONS & USAGE
NITROSTAT CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
NITROSTAT ADVERSE REACTIONS
OVERDOSAGE
Hemodynamic Effects:Methemoglobinemia:
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Nitrostat(Nitroglycerin Sublingual Tablets, USP)
What is NITROSTAT?
What is Angina?
Who should not use NITROSTAT?
-
● very recent heart attack
-
● severe anemia
-
● increased pressure in the head
What should I tell my doctor before taking NITROSTAT?
-
● You are taking any medicines that are used to treat angina, heart failure, or an irregular heartbeat.
-
● You are taking any medicines that reduce blood pressure.
-
● You are taking any diuretics (water pills).
-
● You are taking medications to treat depression or psychiatric illness.
-
● You are taking ergotamine or similar drugs for migraine headaches.
-
● You are taking aspirin.
-
● You are taking the blood thinner medicine heparin.
-
● You are taking any medicines for erectile dysfunction.
-
● You are pregnant or plan to become pregnant.
-
● You are breastfeeding.
-
● Do not chew, crush, or swallow NITROSTAT tablets.
-
● You should sit down when taking NITROSTAT tablets and use caution when you stand up. This eliminates the possibility of falling due to lightheadedness or dizziness.
-
● One tablet should be dissolved under the tongue or in the oral cavity at the first sign of chest pain.
-
● The dose may be repeated approximately every 5 minutes, until the chest pain is relieved.
-
● If the pain persists after a total of 3 tablets in a 15-minute period, or is different than you typically experience, call your doctor or seek emergency help.
-
● NITROSTAT may be used 5 to 10 minutes prior to activities that might cause chest pain.
-
● You may feel a burning or tingling sensation in your mouth when you take NITROSTAT.
-
● Do not breastfeed. It is not known if NITROSTAT will pass through your milk.
-
● Do not consume alcohol while taking NITROSTAT, as this can lower your blood pressure.
-
● Do not start any new prescription or non-prescription medicines or supplements, unless you check with your doctor first.
-
● headache
-
● vertigo (a major symptom of balance disorder)
-
● dizziness
-
● weakness
-
● heart palpitations (unusual awareness of the heartbeat)
-
● low blood pressure upon rising from a seated position
-
● nausea and vomiting
-
● sweating
-
● paleness
-
● fainting
-
● flushing (warm or red condition of your skin)
-
● other skin reactions that may be severe
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

NitrostatNitroglycerin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!