Nifedipine
FULL PRESCRIBING INFORMATION: CONTENTS*
- NIFEDIPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS AND METABOLISM
- INDICATIONS & USAGE
- NIFEDIPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- PEDIATRIC USE
- NIFEDIPINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
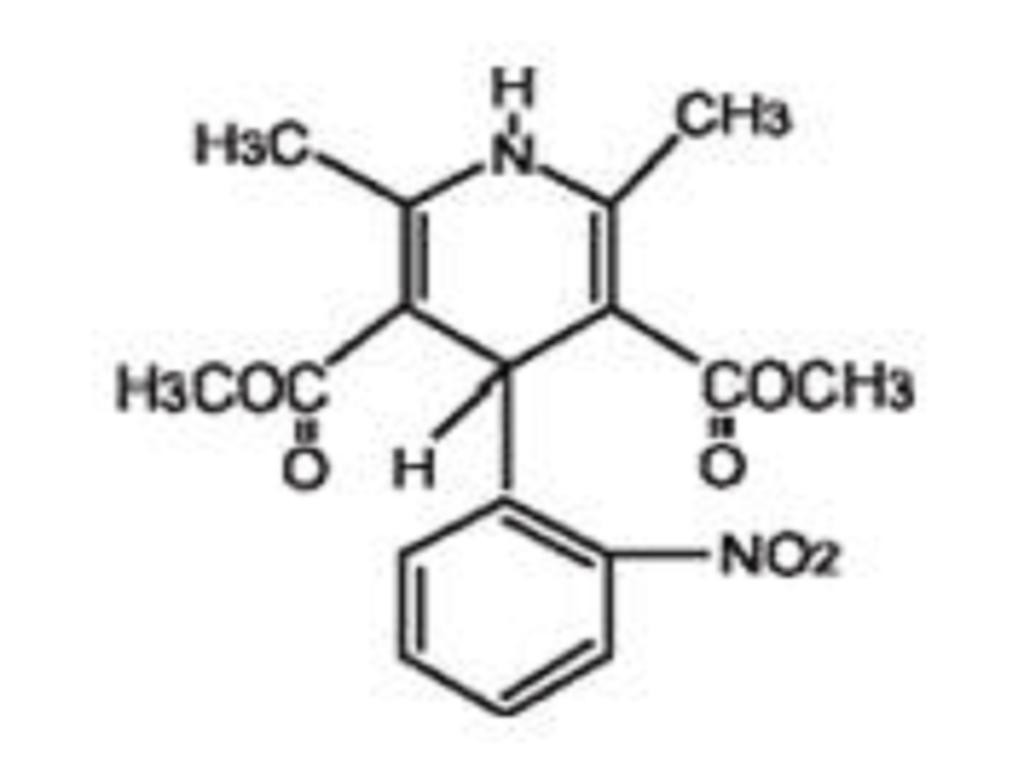
NIFEDIPINE DESCRIPTION
System Components and Performance
CLINICAL PHARMACOLOGY
Mechanism of Action
A) Angina
1) Relaxation and Prevention of Coronary Artery Spasm
2) Reduction of Oxygen Utilization
B) Hypertension
PHARMACOKINETICS AND METABOLISM
Hemodynamics
Electrophysiologic Effects
INDICATIONS & USAGE
I. Vasospastic AnginaII. Chronic Stable Angina (Classical Effort-Associated Angina)
WARNINGS
III. Hypertension
NIFEDIPINE CONTRAINDICATIONS
WARNINGS
Excessive HypotensionIncreased Angina and/or Myocardial Infarction
Beta Blocker Withdrawal
Congestive Heart Failure
Gastrointestinal Obstruction Requiring Surgery
PRECAUTIONS
GeneralHypotension
WARNINGS
Peripheral Edema
INFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
Beta-adrenergic Blocking AgentsINDICATIONS AND USAGEWARNINGS
Long-acting Nitrates
Digitalis
Coumarin Anticoagulants
Cimetidine
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category CPEDIATRIC USE
NIFEDIPINE ADVERSE REACTIONS
PRECAUTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
Co-Administration with Other Antianginal Drugs
PRECAUTIONS: Drug Interactions
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

NifedipineNifedipine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!