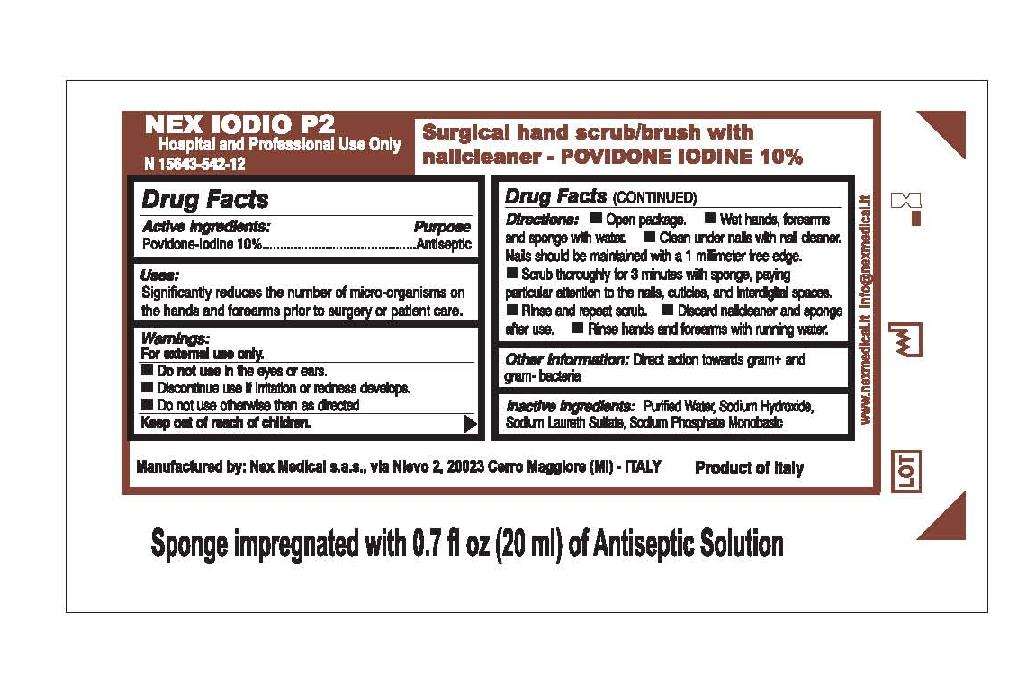
Nex Iodio P2
Nex Medical S.A.S. di Villa Annamaria & C.
Nex Medical S.A.S. di Villa Annamaria & C.
Nex Iodio P2 Povidone-Iodine 10%
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients:
Povidone-Iodine 10 percent
Purpose
Purpose
Antiseptic
Uses
Uses:
Significantly reduces the number of micro-organisms on
the hands and forearms prior to surgery or patient care.
Warnings:
For external use only.
- Do not use in the eyes or ears.
- Discontinue use if irritation or redness develops.
- Do not use otherwise than as directed
Keep out of reach of children.
Directions:
- Open package.
- Wet hands, forearms and sponge with water.
- Clean under nails with nail cleaner.
Nails should be maintained with a 1 millimeter free edge.
- Scrub thoroughly for 3 minutes with sponge, paying particular
attention to the nails, cuticles, and interdigital spaces.
- Rinse and repeat scrub. Discard nailcleaner and sponge after use.
- Rinse hands and forearms with running water.
Other Information:
Direct action towards gram-positive and gram-negative bacteria
Inactive Ingredients:
Purified Water, Sodium Hydroxide,
Sodium Laureth Sulfate, Sodium Phosphate Monobasic
NEX IODIO P2
Hospital and Professional Use Only
Surgical hand scrub/brush with
nailcleaner - POVIDONE IODINE 10%
Manufactured by: Nex Medical s.a.s., via Nievo 2, 20023 Cerro Maggiore (MI) - ITALY
Product of Italy
www.nexmedical.it
info@nexmedical.it
Sponge impregnated with 0.7 fl oz (20 ml) of Antiseptic Solution
Nex Iodio P2Povidone-Iodine SPONGE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||