Neutrogena SkiniD
Neutrogena SkiniD Personalized Acne Solution Toner
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- Questions or comments?
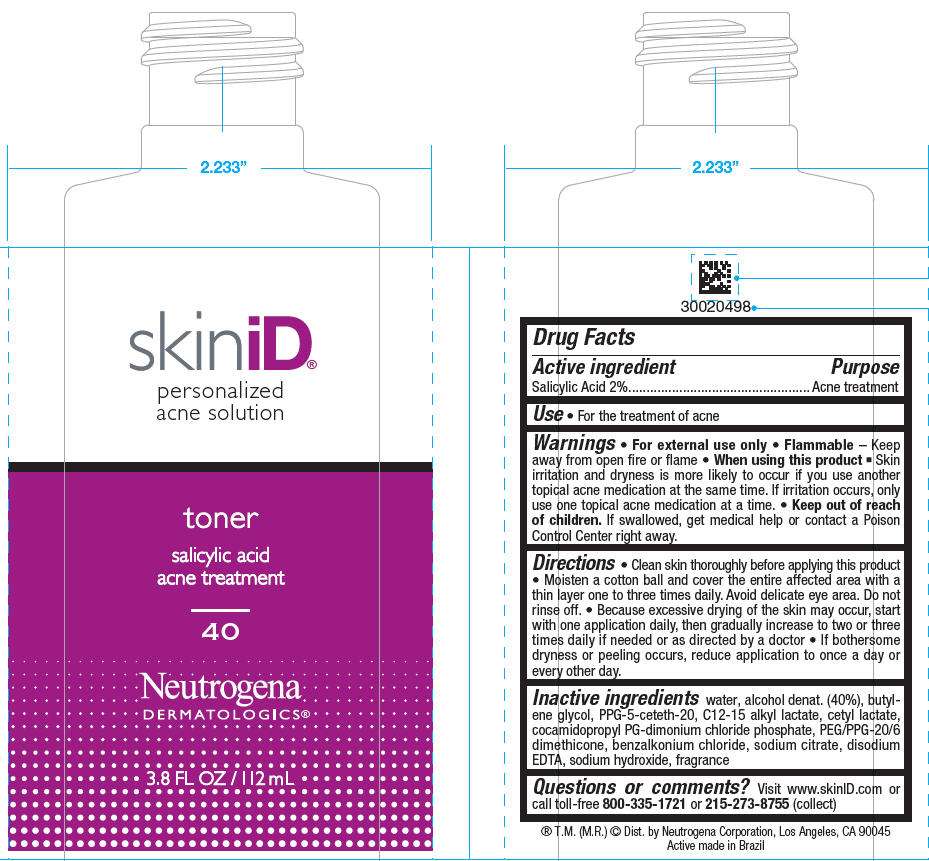
- PRINCIPAL DISPLAY PANEL - 112 mL Bottle Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Salicylic Acid 2%
Purpose
Acne treatment
Use
- For the treatment of acne
Warnings
- For external use only
- Flammable – Keep away from open fire or flame
-
When using this product
- Skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Clean skin thoroughly before applying this product
- Moisten a cotton ball and cover the entire affected area with a thin layer one to three times daily. Avoid delicate eye area. Do not rinse off.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive ingredients
water, alcohol denat. (40%), butylene glycol, PPG-5-ceteth-20, C12-15 alkyl lactate, cetyl lactate, cocamidopropyl PG-dimonium chloride phosphate, PEG/PPG-20/6 dimethicone, benzalkonium chloride, sodium citrate, disodium EDTA, sodium hydroxide, fragrance
Questions or comments?
Visit www.skinID.com or call toll-free 800-335-1721 or 215-273-8755 (collect)
Dist. by Neutrogena Corporation, Los Angeles, CA 90045
PRINCIPAL DISPLAY PANEL - 112 mL Bottle Label
skiniD
®
personalized
acne solution
toner
salicylic acid
acne treatment
40
Neutrogena
DERMATOLOGICS®
3.8 FL OZ / 112 mL
Neutrogena SkiniDSalicylic Acid LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!