Neutrogena Nourishing Eye Quad
Neutrogena Nourishing Eye Quad SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Inactive Ingredients
- Warning
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 4.11 g Butter Crème Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Tea Biscuit Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Copper Glow Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Sea Shell Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Blue Smoke Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Garden Party Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Moonlit Violet Label
- PRINCIPAL DISPLAY PANEL - 4.11 g Vintage Wine Label
FULL PRESCRIBING INFORMATION
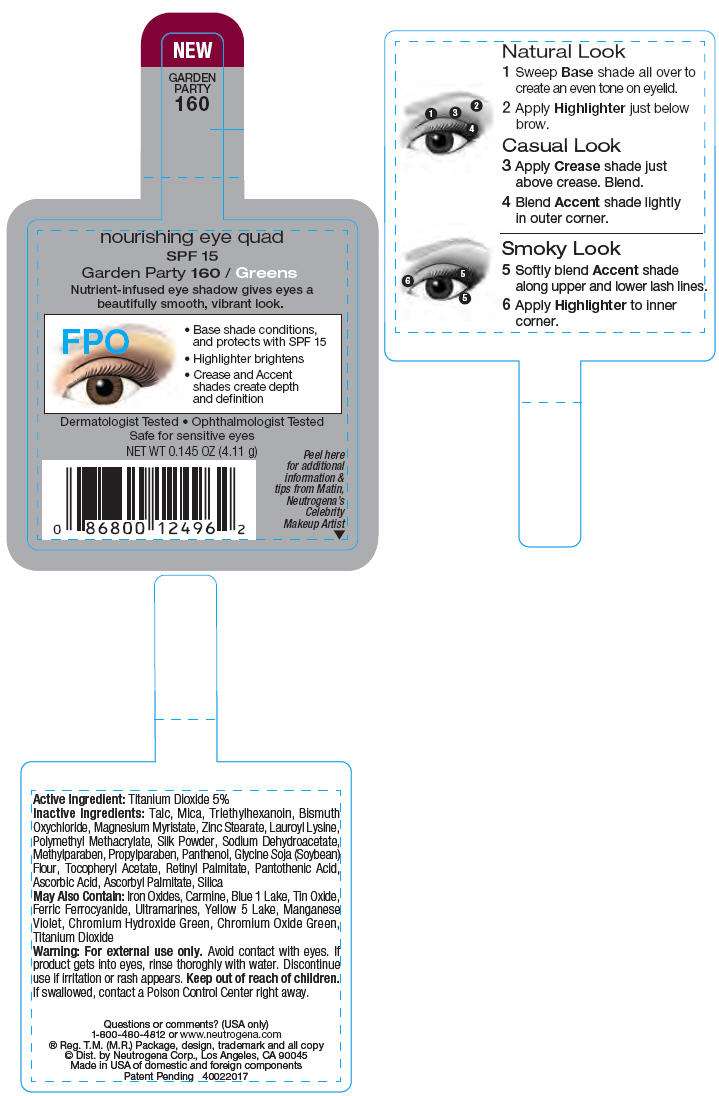
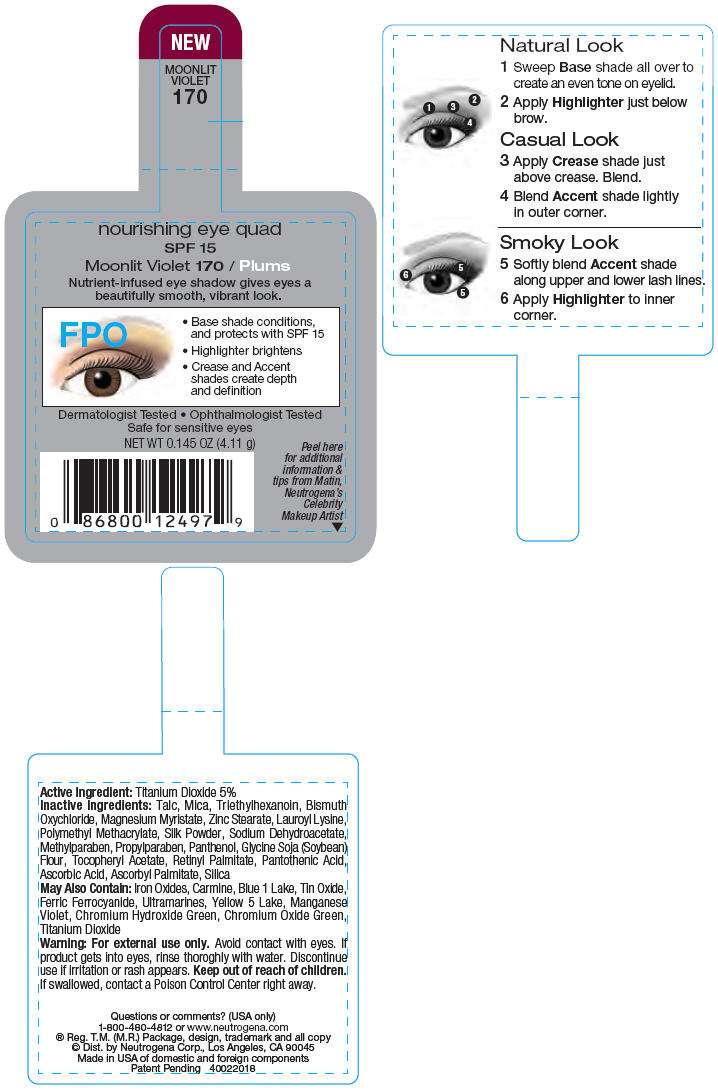
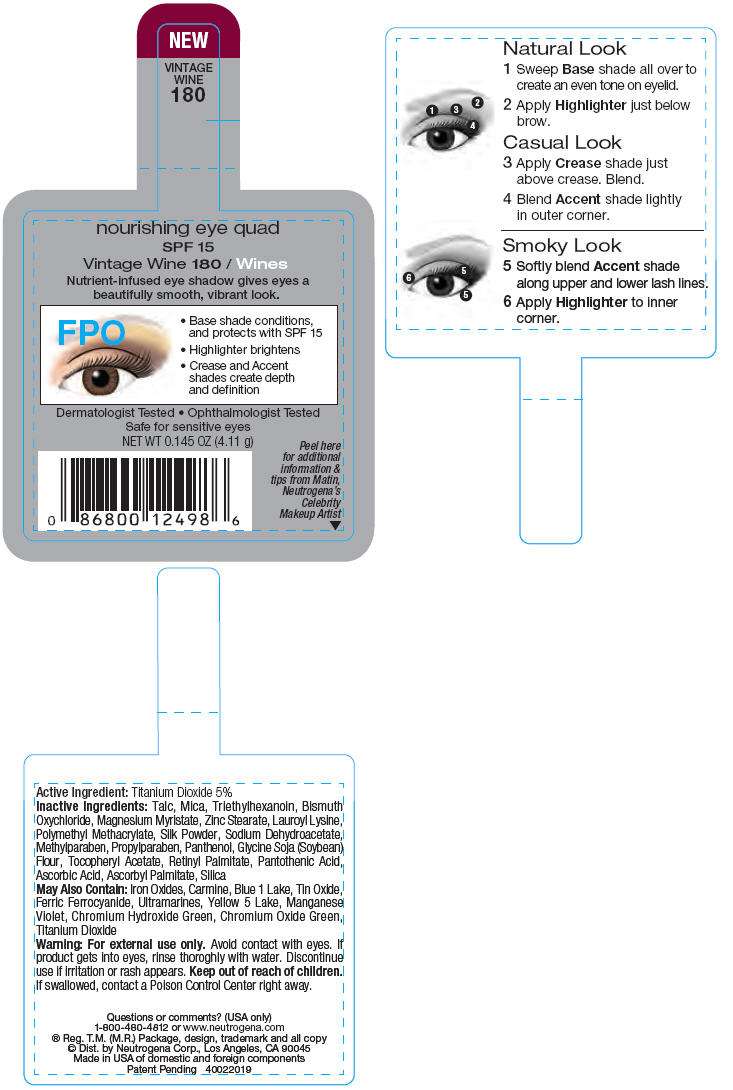
Active Ingredient
Titanium Dioxide 5%
Inactive Ingredients
Talc, Mica, Triethylhexanoin, Bismuth Oxychloride, Magnesium Myristate, Zinc Stearate, Lauroyl Lysine, Polymethyl Methacrylate, Silk Powder, Sodium Dehydroacetate, Methylparaben, Propylparaben, Panthenol, Glycine Soja (Soybean) Flour, Tocopheryl Acetate, Retinyl Palmitate, Pantothenic Acid, Ascorbic Acid, Ascorbyl Palmitate, Silica
May Also Contain
Iron Oxides, Carmine, Blue 1 Lake, Tin Oxide, Ferric Ferrocyanide, Ultramarines, Yellow 5 Lake, Maganese Violet, Chromium Hydroxide Green, Chromium Oxide Green, Titanium Dioxide
Warning
For external use only. Avoid contact with eyes. If product gets into eyes, rinse thoroughly with water. Discontinue use if irritation or rash appears.
Keep out of reach of children. If swallowed, contact a Poison Control Center right away.
Questions or comments?
(USA only) 1-800-480-4812 or www.neutrogena.com
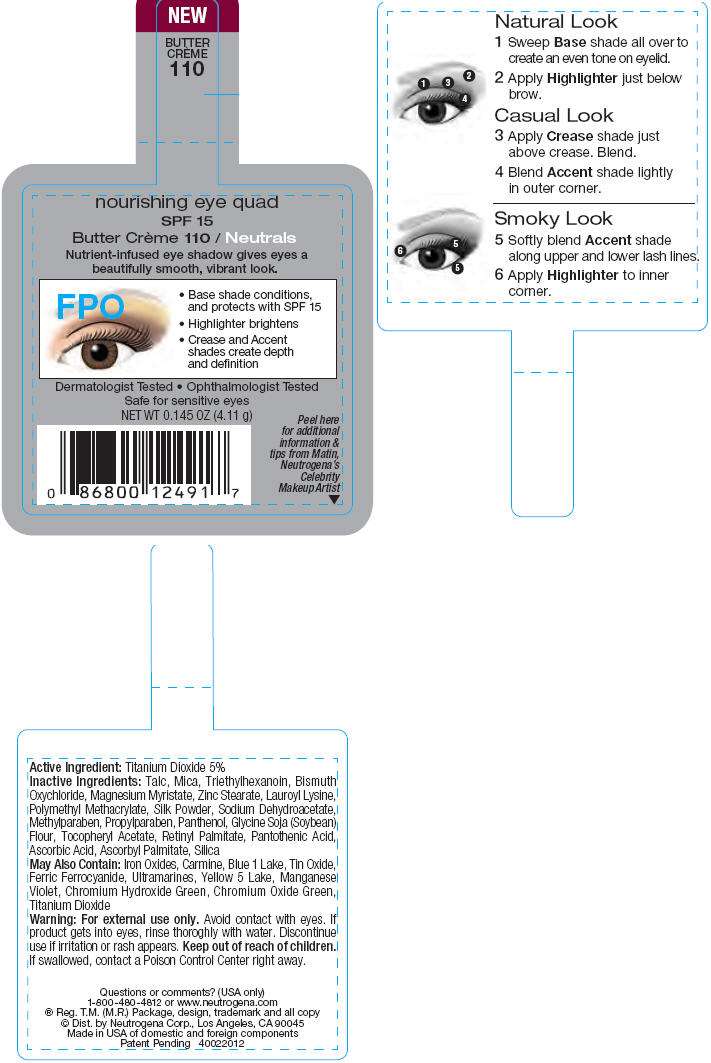
PRINCIPAL DISPLAY PANEL - 4.11 g Butter Crème Label
NEW
BUTTER
CRÈME
110
nourishing eye quad
SPF 15
Butter Crème 110 / Neutrals
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
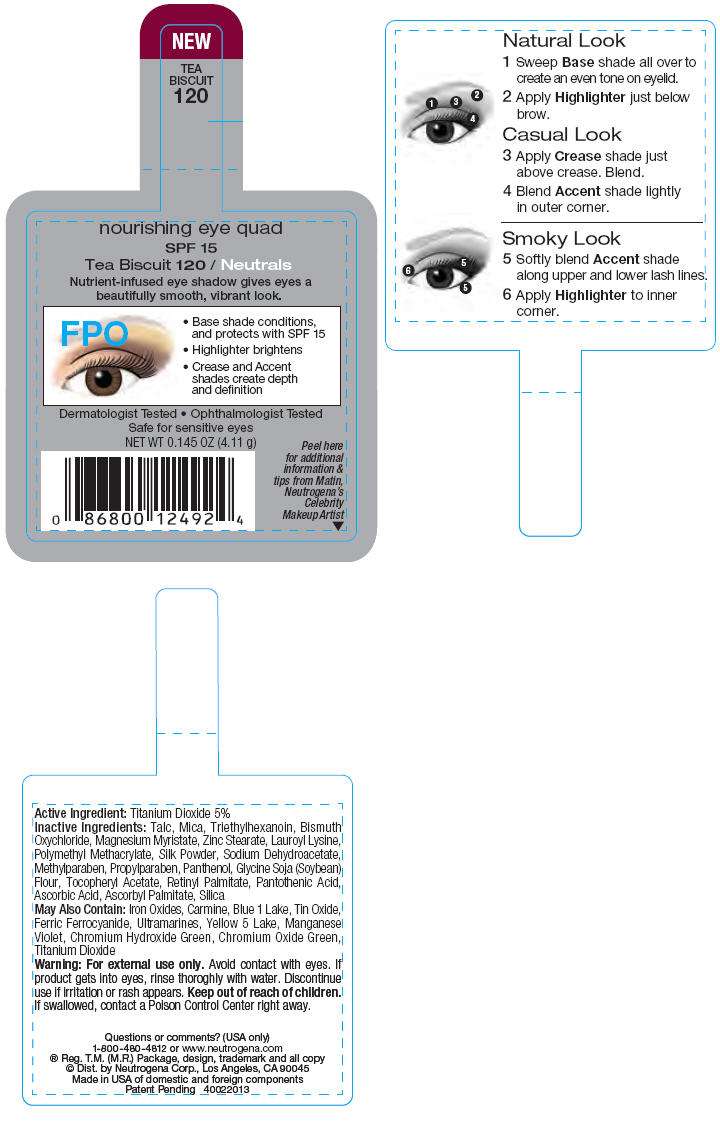
PRINCIPAL DISPLAY PANEL - 4.11 g Tea Biscuit Label
NEW
TEA
BISCUIT
120
nourishing eye quad
SPF 15
Tea Biscuit 120 / Neutrals
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
PRINCIPAL DISPLAY PANEL - 4.11 g Copper Glow Label
NEW
COPPER
GLOW
130
nourishing eye quad
SPF 15
Copper Glow 130 / Neutrals
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist

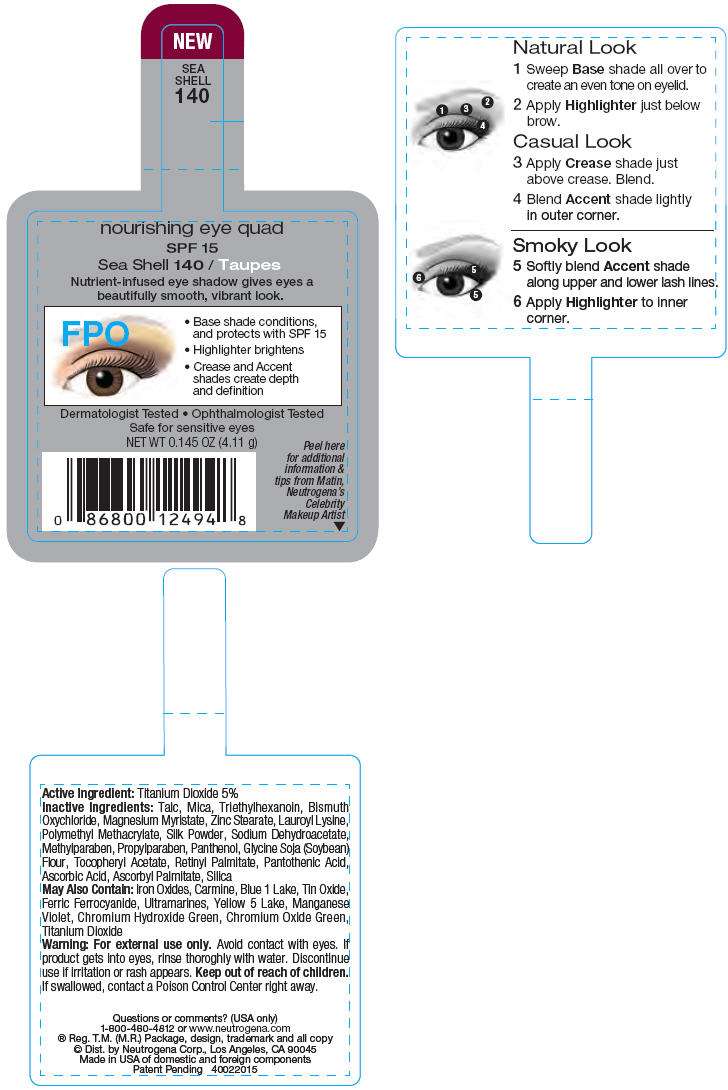
PRINCIPAL DISPLAY PANEL - 4.11 g Sea Shell Label
NEW
SEA
SHELL
140
nourishing eye quad
SPF 15
Sea Shell 140 / Taupes
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
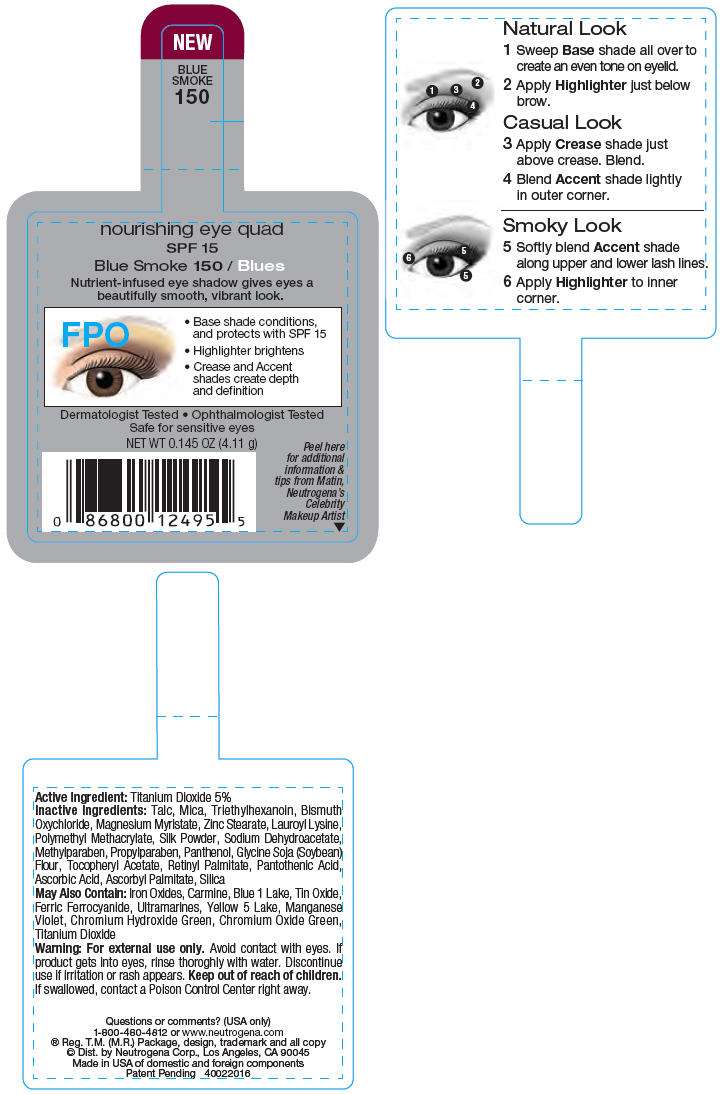
PRINCIPAL DISPLAY PANEL - 4.11 g Blue Smoke Label
NEW
BLUE
SMOKE
150
nourishing eye quad
SPF 15
Blue Smoke 150 / Blues
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
PRINCIPAL DISPLAY PANEL - 4.11 g Garden Party Label
NEW
GARDEN
PARTY
160
nourishing eye quad
SPF 15
Garden Party 160 / Greens
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
PRINCIPAL DISPLAY PANEL - 4.11 g Moonlit Violet Label
NEW
MOONLIT
VIOLET
170
nourishing eye quad
SPF 15
Moonlit Violet 170 / Plums
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
PRINCIPAL DISPLAY PANEL - 4.11 g Vintage Wine Label
NEW
VINTAGE
WINE
180
nourishing eye quad
SPF 15
Vintage Wine 180 / Wines
Nutrient-infused eye shadow gives eyes a
beautifully smooth, vibrant look.
FPO
- Base shade conditions,
and protects with SPF 15 - Highlighter brightens
- Crease and Accent
shades create depth
and definition
Dermatologist Tested • Ophthalmologist Tested
Safe for sensitive eyes
NET WT 0.145 OZ (4.11 g)
Peel here
for additional
information &
tips from Matin,
Neutrogena's
Celebrity
Makeup Artist
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
Neutrogena Nourishing Eye QuadTitanium Dioxide POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||