Neutrogena Corporation
MoistureShine Lipstick SPF 20
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Active ingredient
Purpose
|
Active Ingredients
|
Purpose
|
| Octinoxate 5% |
Sunscreen |
| Octisalate 2.5% |
Sunscreen |
| Titanium Dioxide 1.1% |
Sunscreen |
Neutrogena MoistureShine SPF20 Uses
- Helps prevent sunburn.
- Higher SPF gives more sunburn protection.
- Provides moderate protection against sunburn.
Warnings
For external use only.
When using this product
- Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
- Rash or irritation develops and lasts.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Diisostearyl Malate ,Octyldodecanol, Triisostearin, Dipentaerythrityl Hexahydroxystearate, Microcrystalline Wax, Polyethylene, VP/Hexadecene Copolymer, Paraffin , Astrocaryum Murumuru Butter, Polybutene, Caprylic/Capric Triglyceride, C10-30 Cholesterol/Lanosterol Ester HDI/Trimethylol Hexyllactone Crosspolymer, Ozokerite, Ethylhexyl Palmitate, Dicalcium Phosphate, Stearalkonium Bentonite, Methicone, Tribehenin, Propylene Carbonate , Alumina, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Sorbitan Isostearate, Silica, Fragrance, Tocopherol, Palmitoyl Oligopeptide
May Also Contain: Mica, Titanium Dioxide, Iron Oxides, Carmine, Red 6, Red 7, Red 7 Lake, Red 30 Lake, Yellow 5 Lake, Yellow 6 Lake, Blue 1 Lake, Red 27, Red 28 Lake
Questions?
Call 1-800-480-4812 or visit www.neutrogena.com
PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
ANGEL'S
BLUSH
100
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
THINK
PINK
110
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
TICKLED
PINK
120
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
ANYTHING
ROSE
200
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
JUST
WHISPER
210
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
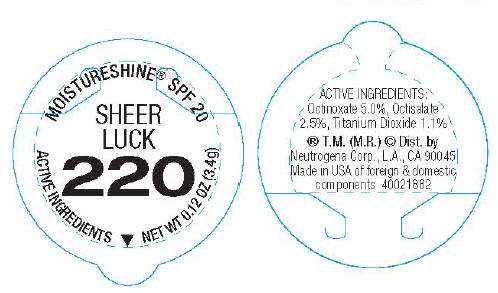
MOISTURESHINE® SPF 20
SHEER
LUCK
220
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)
PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
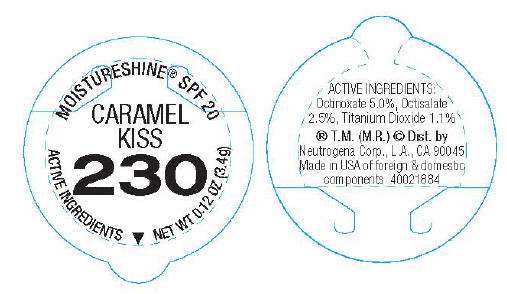
MOISTURESHINE® SPF 20
CARAMEL
KISS
230
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)
PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
NUDE
BLUSH
240
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
RUM
RAISIN
250
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
PINCH
OF CINN
260
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
MAUVE
ON
300
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
PLAYFUL
PLUM
310
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
PLUM
PARADISE
320
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
BERRY
BURST
400
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
CHERRY
TWIST
410
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
POPPY
RED
420
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
POMEGRANATE
430
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
ELECTRIC
CURRANT
440
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
CORAL
DREAM
500
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

PRINCIPAL DISPLAY PANEL - 3.4 g Tube Label
MOISTURESHINE® SPF 20
PEACH-
A-BOO
510
ACTIVE INGREDIENTS
NET WT 0.12 OZ (3.4g)

Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-331 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-331-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-332 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-332-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-333 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-333-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-334 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-334-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-335 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-335-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-336 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-336-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-337 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-337-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-338 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-338-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-339 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-339-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-340 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-340-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-341 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-341-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-342 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-342-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-343 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-343-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-344 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-344-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-345 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-345-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-346 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-346-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-347 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-347-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-348 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-348-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-349 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-349-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|
Neutrogena MoistureShine SPF20
Octinoxate, Octisalate, and Titanium Dioxide LIPSTICK
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:10812-350 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:10812-350-01 |
3.4 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2010-02-15 |
|
|



















