NERVE TONIC STRESS RELIEF
NERVE TONIC™ STRESS RELIEF
FULL PRESCRIBING INFORMATION: CONTENTS*
- NERVE TONIC STRESS RELIEF Uses
- Warnings
- Directions
- Inactive Ingredients
- Questions?
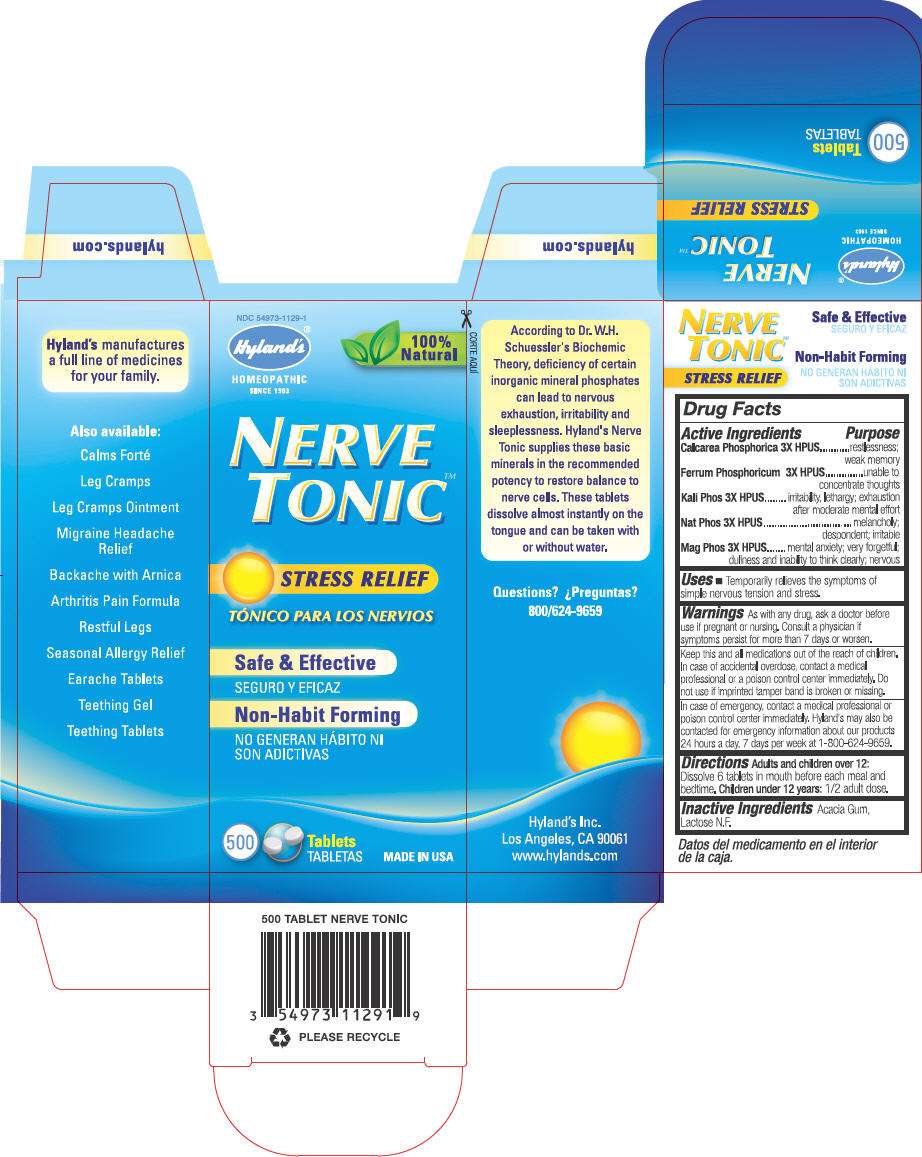
- PRINCIPAL DISPLAY PANEL - 500 Tablet Bottle Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Purpose
| Active Ingredients | Purpose |
|---|---|
| Calcarea Phosphorica 3X HPUS | restlessness; weak memory |
| Ferrum Phosphoricum 3X HPUS | unable to concentrate thoughts |
| Kali Phos 3X HPUS | irritability, lethargy; exhaustion after moderate mental effort |
| Nat Phos 3X HPUS | melancholy; despondent; irritable |
| Mag Phos 3X HPUS | mental anxiety; very forgetful; dullness and inability to think clearly; nervous |
NERVE TONIC STRESS RELIEF Uses
- Temporarily relieves the symptoms of simple nervous tension and stress.
Warnings
As with any drug, ask a doctor before use if pregnant or nursing.
Consult a physician if symptoms persist for more than 7 days or worsen.
Keep this and all medications out of the reach of children. In case of accidental overdose, contact a medical professional or a poison control center immediately.
Do not use if imprinted tamper band is broken or missing.
In case of emergency, contact a medical professional or poison control center immediately. Hyland's may also be contacted for emergency information about our products 24 hours a day, 7 days per week at 1-800-624-9659.
Directions
Adults and children over 12
Dissolve 6 tablets in mouth before each meal and bedtime.
Children under 12 years
½ adult dose.
Inactive Ingredients
Acacia Gum, Lactose N.F.
Questions?
800/624-9659
PRINCIPAL DISPLAY PANEL - 500 Tablet Bottle Carton
NDC 54973-1129-1
Hyland's ®
HOMEOPATHIC
SINCE 1903
100%
Natural
NERVE
TONIC™
STRESS RELIEF
Safe & Effective
Non-Habit Forming
500
Tablets
MADE IN USA
NERVE TONIC STRESS RELIEFCALCIUM PHOSPHATE, IRON, POTASSIUM PHOSPHATE, UNSPECIFIED FORM, MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE, and SODIUM PHOSPHATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||