Neova
Neova Breakout Control - Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Neova Uses
- Warnings
- When using this product
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Image of Box Label
FULL PRESCRIBING INFORMATION
Active Ingredients
Salicylic Acid 2%
Purpose
Acne Treatment
Neova Uses
• For the treatment of acne. • Helps prevent new acne pimples,
blackheads and whiteheads.
Warnings
For external use only
When using this product
Avoid contact with eyes. If contact occurs, flush
thoroughly with water. Using other topical acne medications at the same time
or immediately following use of this product may increase dryness or
irritation of the skin. If this occurs, only one medication should be used unless
directed by a doctor. Do not insert in ear canal.
Keep out of reach of children.
If swallowed, contact a Poison Control
Center immediately or get medical help right away.
Directions
• Snap the tip at the color ring. • Allow formula to flow down
to the opposite tip. • Apply formula with the saturated tip to affected area.
Other Information
Avoid storing at excessive heat.
Inactive Ingredients
Allantoin, Hamamelis Virginiana (Witch Hazel)
Water, Aloe Barbadensis Leaf Extract, Isoceteth-20, Methyl Gluceth-20, PEG-6,
Propylene Glycol, Sodium Hydroxide, Sodium PCA, Sorbitol, Tetrasodium EDTA,
Water (Aqua).
Image of Box Label
BreakOutControlBoxLabel112611.jpg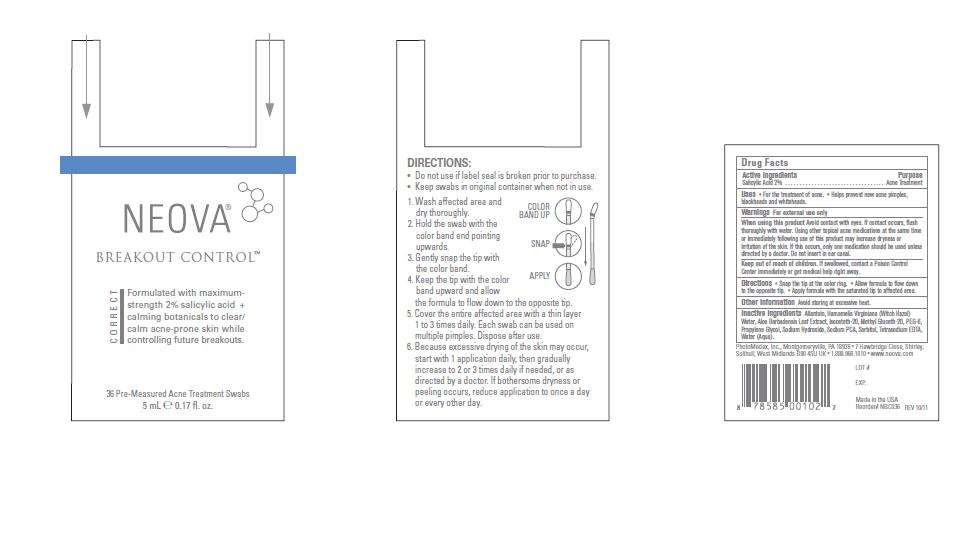
NeovaSalicylic Acid SWAB
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||