Neova DNA Damage Control - Silc Sheer
Neova DNA Damage Control - Silc Sheer SPF 45 - Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredients
- Purpose
- Neova DNA Damage Control - Silc Sheer Uses
- Warnings
- When using this product:
- Stop use and ask a doctor if:
- Keep out of reach of children:
- Directions
- Other Information
- Inactive Ingredients
- Image of box and label
FULL PRESCRIBING INFORMATION
Active Ingredients
Octinoxate 6.5%, Titanium Dioxide 3.5%
Purpose
Sunscreen
Neova DNA Damage Control - Silc Sheer Uses
• Helps prevent sunburn. • Higher SPF gives
more sunburn protection. • Retains SPF after 80 minutes
of activity in the water or perspiring.
Warnings
For external use only
When using this product:
Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if:
Rash or irritation develops and lasts.
Keep out of reach of children:
If swallowed, contact a Poison Control Center immediately or get medical help right away.
Directions
• Apply every morning to face, neck
and décolletage. • Reapply as needed or after towel
drying, swimming or perspiring.
• Children under 6 months of age: ask a doctor.
Other Information
High protection sun product. Sun Alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Serious side effects associated with use of this product may be reported to this number: 888-966-1010.
Inactive Ingredients
Camellia Sinensis Leaf
Extract, Aloe Barbadensis Extract, Octyl Stearate,
Cyclomethicone, Isopropyl Palmitate, Helianthus Annuus
(Sunflower) Seed Oil, Lauryl PEG-9 Polydimethylsiloxyethyl
Dimethicone, Micrococcus Lysate, Plankton
Extract, Xanthophyll, Rosmarinus Officinalis (Rosemary)
Leaf Extract, Squalane, Lecithin, Silica, Hydrated Silica,
Microcrystalline Wax, Cetyl Dimethicone, Hydrogenated
Castor Oil, Sodium Chloride, Phenoxyethanol, Iodopropynyl
Butylcarbamate, Melanin, Caramel, Iron Oxides, CI 77499,
CI 77491, CI 77492.
Image of box and label
DNADControlSHEERBox.jpg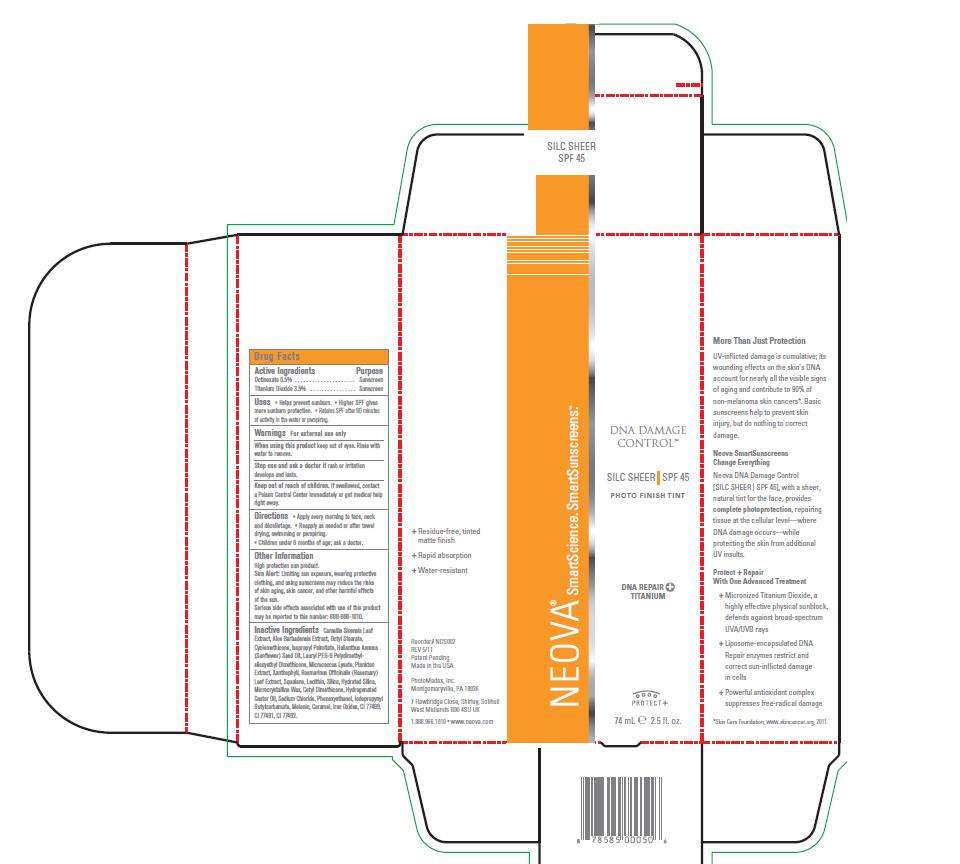 DNADControlSHEERLabel.jpg
DNADControlSHEERLabel.jpg
Neova DNA Damage Control - Silc SheerOctinoxate, Titanium Dioxide EMULSION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||