NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE
Fera Pharmaceuticals
Fera Pharmaceuticals
NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE OPHTHALMIC OINTMENT USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE INDICATIONS AND USAGE:
- NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE ADVERSE REACTIONS:
- NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- Principal Display Panel - Carton Label
- Principal Display Panel - Tube Label
FULL PRESCRIBING INFORMATION
DESCRIPTION:
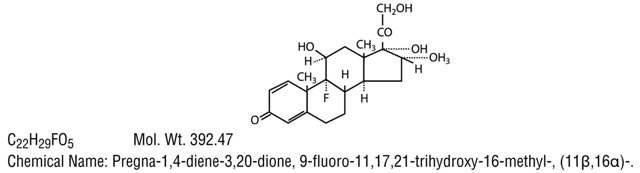
Neoymcin and Polymyxin B Sulfates and Dexamethasone Ophthalmic Ointment USP is a multiple dose anti-infective steroid combination in a sterile ointment for topical application. The active ingredient dexamethasone, is represented by the following structural formula:
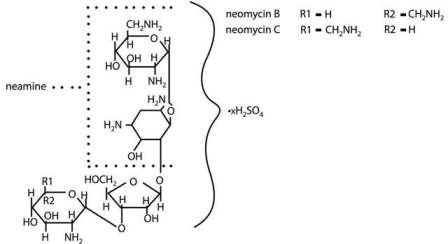
Neomycin Sulfate is the sulfate salt of neomycin B and C which are produced by the growth of Streptomyces fradiae Waksman (Fam. Streptomycetaceae). It has a potency equivalent to not less than 600 micrograms of neomycin base per milligram, calculated on an anhydrous basis. The structural formula are:
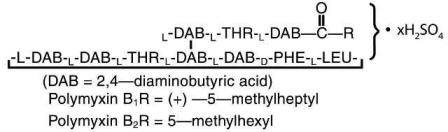
Polymyxin B sulfate is the sulfate salt of polymyxin B1 and B2 which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillaceae). It has a potency of not less than 6,000 polymyxin B units per milligram, calculated on an anhydrous basis. The structural formula are:
Each gram of ointment contains: ACTIVES: Dexamethasone 1 mg (0.1%), Neomycin Sulfate equivalent to Neomycin 3.5 mg base, Polymyxin B Sulfate 10,000 units in a base containing INACTIVES: White Petrolatum and Mineral Oil.
CLINICAL PHARMACOLOGY:
INDICATIONS AND USAGE:
For steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where bacterial infection or risk of bacterial ocular infection exists.
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitises is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns; or penetration of foreign bodies.
The use of a conbination drug with an anti-infective component is indicated where the risk of infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drugs in this product is active against the following common bacterial eye pathogens: Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Klebsiella/Enterobacter species, Niesseria species, and Pseudomonas aeruginosa.
This product does not provide adequate coverage against: Serratia marcescens and Streptococci, including Streptococcus pneumoniae.
CONTRAINDICATIONS:
Epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and many other viral diseases of the cornea and conjunctiva. Mycobacterial infection of the eye. Fungal diseases of ocular structures. Hypersensitivity to a component of the medication. (Hypersensitivity to the antibiotic component occurs at a higher rate than for the other components.)
The use of these combinations is always contraindicated after uncomplicated removal of a corneal foreign body.
WARNINGS:
NOT FOR INJECTION. Do not touch tube tip to any surface, as this may contaminate the contents. Prolonged use may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. In acute purulent conditions of the eye, steroids may mask infection or enhance existing infection. If these products are used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients.
Products containing neomycin sulfate may cause cutaneous sensitization. Employment of steroid medication in the treatment of herpes simplex requires great caution.
PRECAUTIONS:
The initial prescription and renewal of the medication order beyond 8 g should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy and, where appropriate, flourescein staining. The possibility of persistent fungal infections of the cornea should be considered after prolonged steroid dosing. Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial ocular infection.
PREGNANCY:
Pregnancy Category C: Dexamethasone has been shown to be teratogenic in mice and rabbits following topical ophthalmic application in multiples of the therapeutic dose.
In the mouse, corticosteroids produce fetal resorptions and a specific abnormality, cleft palate. In the rabbit, corticosteroids have produced fetal resorptions and multiple abnormalities involving the head, ears, limbs, palate, etc.
There are no adequate or well-controlled studies in pregnant women. Neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be observed carefully for signs of hypoadrenalism.
NURSING MOTHERS:
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment is administered to a nursing woman.
PEDIATRIC USE:
Safety and effectiveness in pediatric patients have not been established.
GERIATRIC USE:
No overall clinical differences in safety or effectiveness have been observed between the elderly and other adult patients.
ADVERSE REACTIONS:
Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination. Exact incidence figures are not available since no denominator of treated patients is available.
Reactions occurring most often from the presence of the anti-infective ingredients are allergic sensitizations. The reactions due to the steroid component are: elevation of intraocular pressure (IOP) with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
Secondary Infection: The development of the secondary infection has occurred after use of combination containing steroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion must be considered in any persistent corneal ulceration where steroid treatment has been used. Secondary bacterial ocular infection following suppression of host responses also occurs.
To report SUSPECTED ADVERSE REACTIONS, contact Fera Pharmaceuticals, LLC at (414) 434-6604, Monday-Friday 9am-5pm EST, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION:
Apply a small amount into the conjunctival sac(s) up to three or four times daily.
How to Apply neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment:
- Tilt your head back.
- Place a finger on your cheek just under your eye and gently pull down until a "V" pocket is formed between your eyeball and your lower lid.
- Place a small amount (about 1/2 inch) of neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment in the "V" pocket. Do not let the tip of the tube touch your eye.
- Look downward before closing your eye.
HOW SUPPLIED:
Neomycin and polymyxin B sulfates and dexamethasone ophthalmic ointment USP is supplied in 3.5 g (1/8 oz) sterile tamper evident tubes with ophthalmic tip.
NDC 48102-003-35
ooooFERA
Mfd. for:
Fera Pharmaceuticals, LLC
Locust Valley, NY 11560
Principal Display Panel - Carton Label

NDC 48102-003-35
Rx only
FERA
NEOMYCIN AND POLYMYXN B
SULFATES AND DEXAMETHASONE
OPHTHALMIC OINTMENT USP
STERILE
Each gram contains: Neomycin
Sulfate (equiv. to 3.5 mg Neomycin
base); Polymyxin B Sulfate 10,000
Units; Dexamethasone 1 mg; in a
base containing White petrolatum and
Mineral Oil.
Net wt. 3.5 g (1/8 oz)
Principal Display Panel - Tube Label

NDC 48102-004-35
FERA
Rx only
NEOMYCIN AND
POLYMYXIN B
SULFATES AND
DEXAMETHASONE
OPHTHALMIC OINTMENT
USP STERILE
USUAL DOSAGE:
See package insert for full
prescribing information.
See crimp for Lot No.
and Exp. Date.
KEEP TIGHTLY CLOSED.
Mfd for:
Fera Pharmaceuticals, LLC
Locust Valley, NY 11560
Each gram contains:
Neoymcin Sulfate (equiv. to
3.5 mg Neomycin base);
Polymyxin B Sulfate 10,000
units; Dexamethasone 1 mg;
in a base containing White
Petrolatum and Mineral Oil.
Store at 20o to 25oC (68o to
77oF). [See USP Controlled
KEEP OUT OF REACH OF
CHILDREN.
NEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONENEOMYCIN AND POLYMYXIN B SULFATES AND DEXAMETHASONE OINTMENT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||