Naturals Pomegranate and Mango
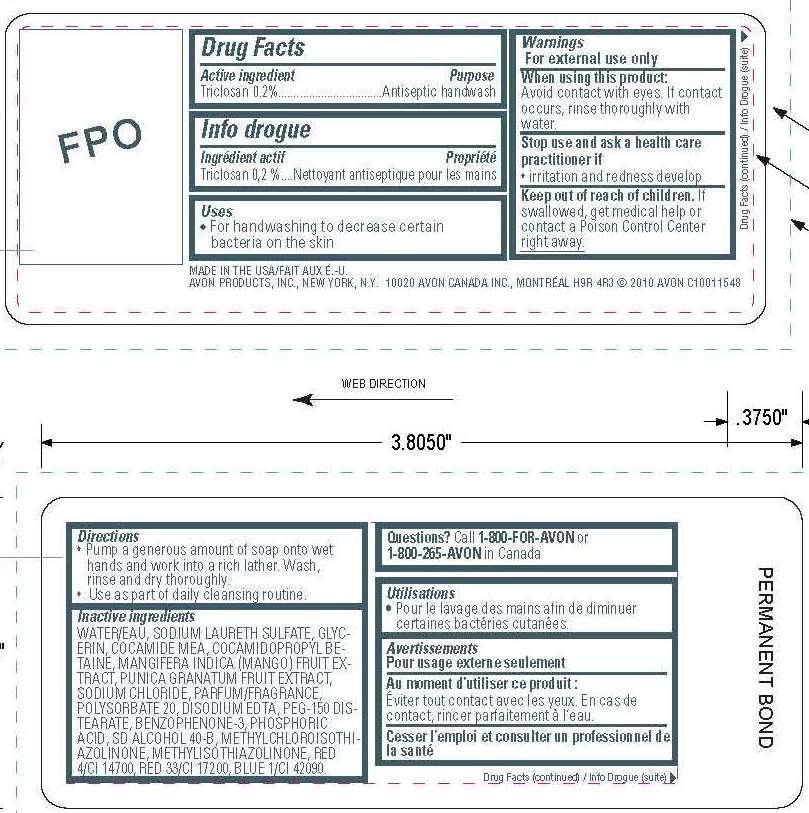
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient Purpose
Triclosan 0.20%..................................Antiseptic handwash
Uses
Uses
- for handwashing to decrease certain bacteria on the skin
Warnings
For external use only
When using this product:
Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
Stop use and ask a healthcare practitioner if
- irritation and redness develop
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Pump a generous amount of soap onto wet hands and work into a rich lather. Wash, rinse and dry thoroughly.
- Use as part of daily cleansing routine.
Inactive Ingredients
WATER/EAU
SODIUM LAURETH SULFATE
GLYCERIN
COCAMIDE MEA
COCAMIDOPROPYL BETAINE
MANGIFERA INDICA (MANGO) FRUIT EXTRACT
PUNICA GRANATUM FRUIT EXTRACT
SODIUM CHLORIDE
PARFUM/FRAGRANCE
POLYSORBATE 20
DISODIUM EDTA
PEG-150 DISTEARATE
BENZOPHENONE-3
PHOSPHORIC ACID
SD ALCOHOL 40-B
METHYLCHLOROISOTHIAZOLINONE
METHYLISOTHIAZOLINONE
RED 4/CI 14700
RED 33/CI 17200
BLUE 1/CI 42090
Questions? Call 1-800-FOR-AVON or 1-800-265-AVON in Canada

Naturals Pomegranate and MangoTRICLOSAN LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||