NARS PRO-PRIME MULTI-PROTECT PRIMER
NARS Pro-Prime Multi-Protect Primer (Full Size)
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
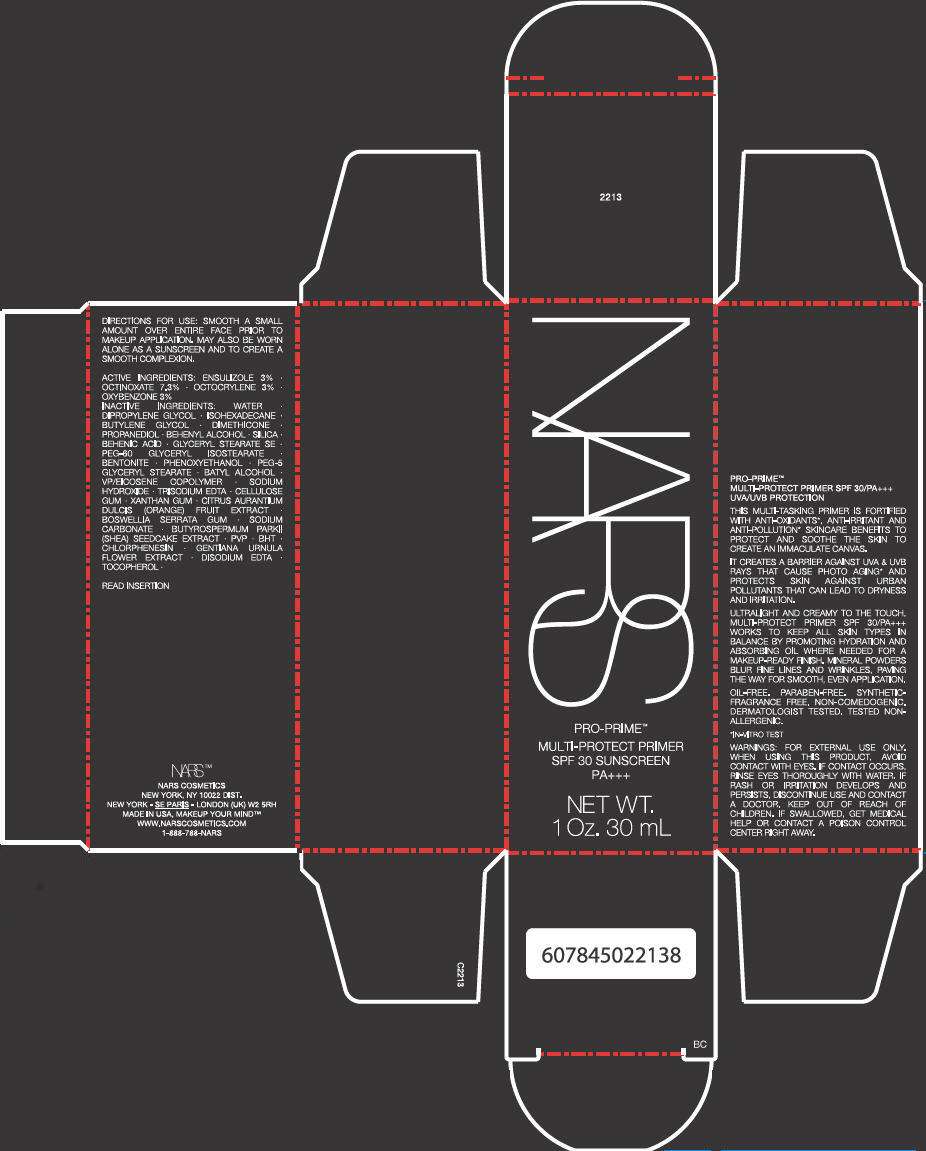
WARNINGS
FOR EXTERNAL USE ONLY.
WHEN USING THIS PRODUCT, AVOID CONTACT WITH EYES. IF CONTACT OCCURS, RINSE EYES THOROUGHLY WITH WATER.
IF RASH OR IRRITATION DEVELOPS AND PERSISTS, DISCONTINUE USE AND CONTACT A DOCTOR.
KEEP OUT OF REACH OF CHILDREN. IF SWALLOWED. GET MEDICAL HELP OR CONTACT A POISON CONTROL CENTER RIGHT AWAY.
ACTIVE INGREDIENTS
ENSULIZOLE 3%, OCTINOXATE 7.3%, OCTOCRYLENE 3%, OXYBENZONE 3%
INACTIVE INGREDIENTS
WATER, DIPROPYLENE GLYCOL, ISOHEXADECANE, BUTYLENE GLYCOL, DIMETHICONE, PROPANEDIOL, BEHENYL ALCOHOL, SILICA, BEHENIC ACID, GLYCERYL STEARATE SE, PEG-60 GLYCERYL ISOSTEARATE, BENTONITE, PHENOXYETHANOL, PEG-5 GLYCERYL STEARATE, BATYL ALCOHOL, VP/EICOSENE COPOLYMER, SODIUM HYDROXIDE, TRISODIUM EDTA, CELLULOSE GUM, XANTHAN GUM, CITRUS AURANTIUM DULCIS (ORANGE) FRUIT EXTRACT, BOSWELLIA SERRATA GUM, SODIUM CARBONATE, BUTYROSPERMUM PARKII (SHEA) SEEDCAKE EXTRACT, PVP, BHT, CHLORPHENESIN, GENTIANA URNULA FLOWER EXTRACT, DISODIUM EDTA, TOCOPHEROL
PRINCIPAL DISPLAY PANEL - 30 mL Carton
NARS
PRO-PRIME™
MULTI-PROTECT PRIMER
SPF 30 SUNSCREEN
PA+++
NET WT. 1 Oz. 30 mL
NARS PRO-PRIME MULTI-PROTECT PRIMEREnsulizole, Octinoxate, Octocrylene, and Oxybenzone EMULSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||